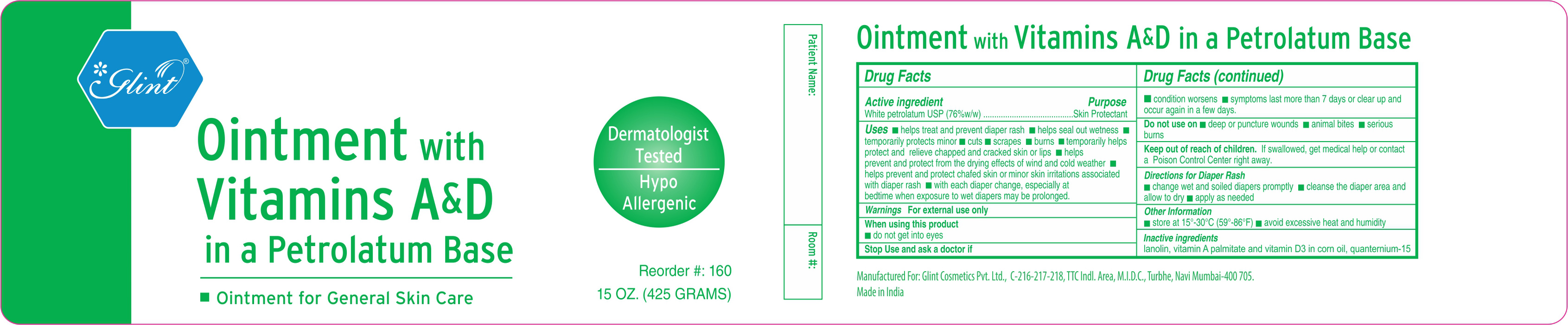
GLINT VITAMIN A D- white petrolatum ointment
Glint Cosmetics Private Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
White petrolatum USP (76% w/w)
Uses
■ Helps treat and prevent diaper rash ■ helps seal out wetness ■ temporarily protects minor ■ cuts ■ scrapes ■ burns ■ temporarily helps protect and relieve chapped and cracked skin or lips ■ helps prevent and protect from the drying effects of wind and cold weather ■ helps prevent and protect chafed skin or minor skin irritations associated with diaper rash ■ with each diaper change, espicially at bedtime when exposure to wet diapers may be prolonged
Warnings
For external use only
When using this product
■ do not get into eyes
Stop use and ask a doctor if
■ condition worsens ■ symptoms last more than 7 days or clear up and occur again in a few days
Do not use on
■ deep or puncture wounds ■ animal bites ■ serious burns
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
For Skin Protection and Diaper rash ■ apply as needed
Additionally when using for Diaper Rash ■ change wet and soiled diapers promptly ■ cleanse the diaper area and allow to dry
Other Information
■ store at 15-30C (59-86F) ■ avoid excessive heat and humidity
Inactive ingredients
lanolin, vitamin A palmitate, vitamin D3 in corn oil, quaternium-15