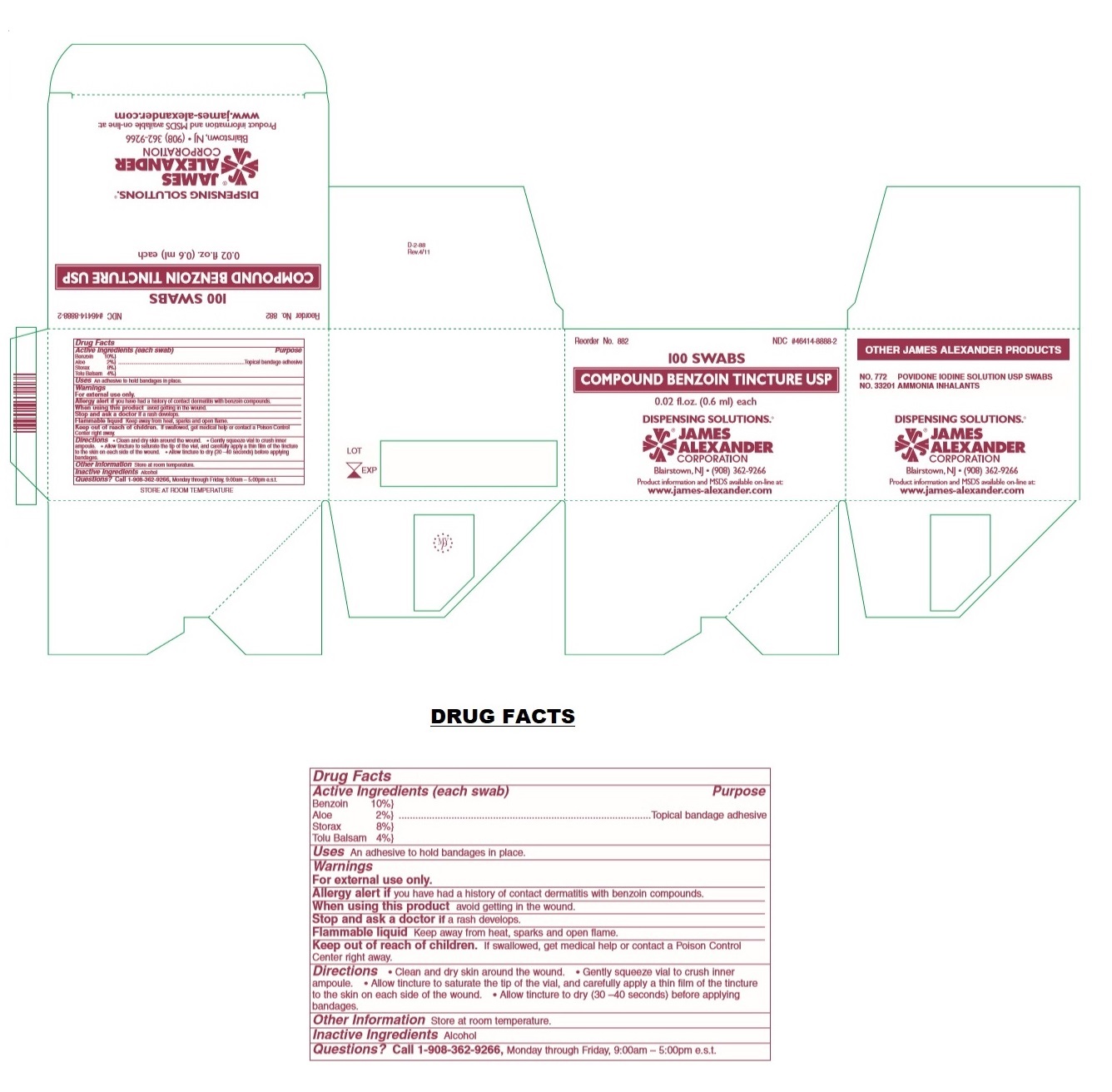
Warnings
For external use only.
Allergy alert if you have had a history of contact dermatitis with benzoin compounds.
When using this product avoid getting in the wound.
Stop and ask a doctor if a rash develops.
Flammable liquid Keep away from heat, sparks and open flame.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• Clean and dry skin around the wound. • Gently squeeze vial to crush inner ampoule. • Allow tincture to saturate the tip of the vial, and carefully apply a thin film of the tincture to the skin on each side of the wound. • Allow tincture to dry (30 –40 seconds) before applying bandages.