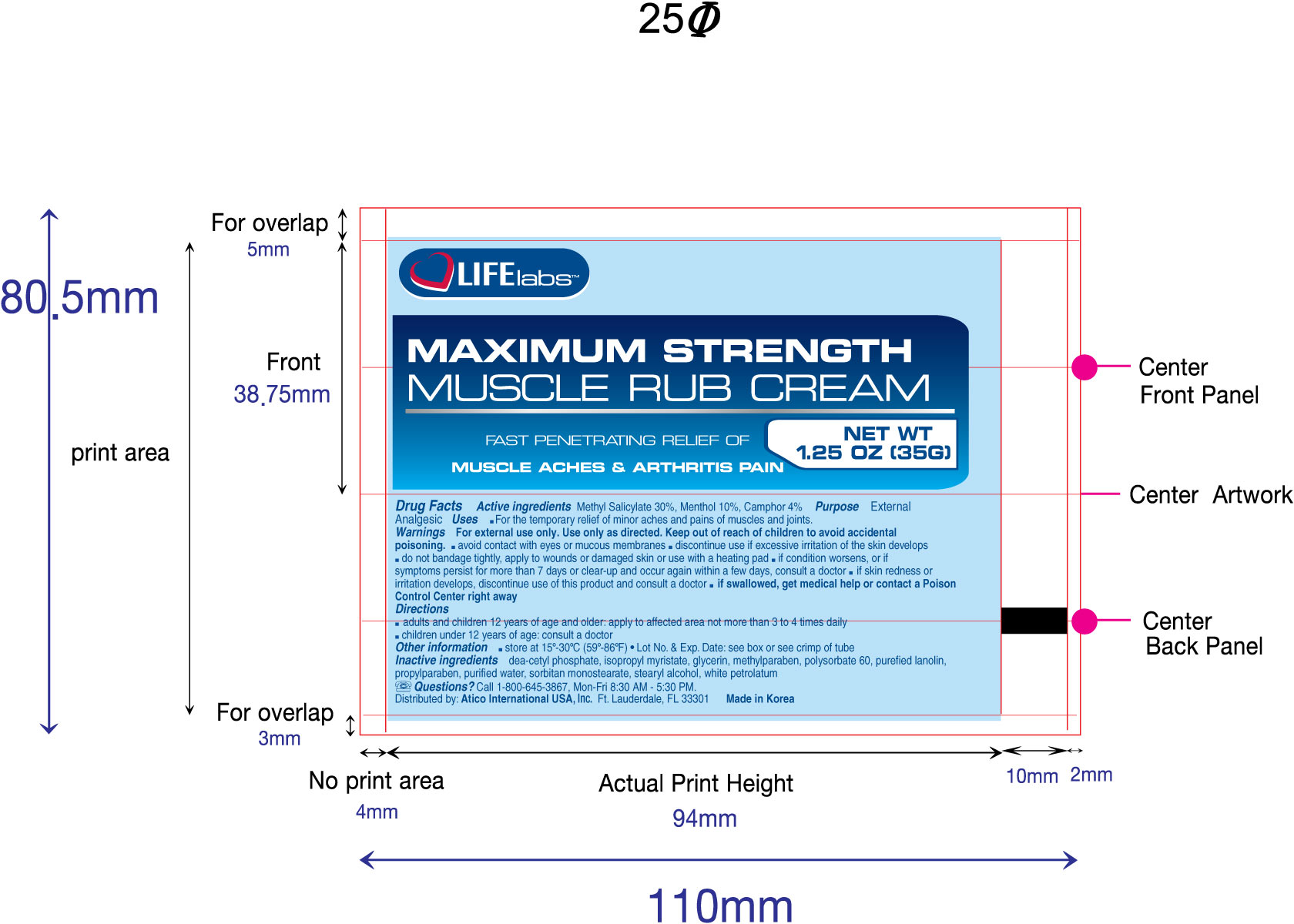
Muscle Rub (Maximum Strength)
Active Ingredients
Methyl Salicylate - 30%, Menthol - 10%, Camphor - 4%
Warnings
For external use only. Use only as directed. Keep out of reach of children to avoid accidental poisoning.
- avoid contact with eyes or mucous membranes.
- discontinue use if excessive irritation of the skin develops.
- do not bandage tightly, apply to wounds, or damaged skin, or use with a heating pad
- if condition worsens, or if symptoms persist for more than 7 days or clear-up and occur again within a few days, consult a doctor
- if skin redness or irritation develops, discontinue use of this product and consult a doctor.
- if swallowed, get medical help or contact a Poison Control Center right away.
Other Information
- Store at controlled room temperature 15o to 30o C (59o to 86oF)
- Lot No. and Exp. Date: see box or see crimp of tube
Directions
- Adults
and children 12 years of age and older: apply to the affected area not
more than 3 to 4 times daily
- Children under 12 years of age: consult a doctor.