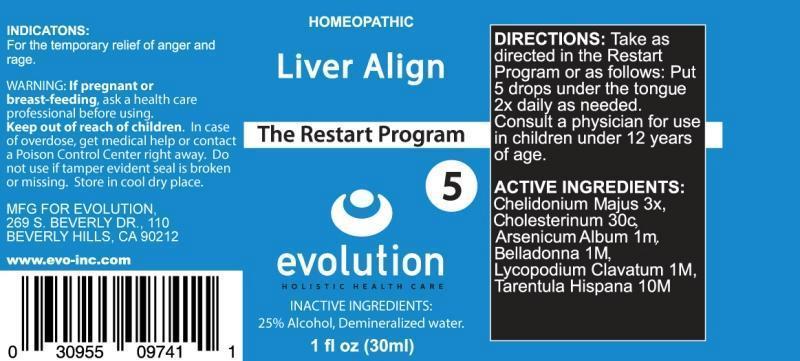
LIVER ALIGN- chelidonium majus, cholesterinum, arsenicum album, belladonna, lycopodium clavatum, tarentula hispana liquid
Evolution
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS:
CHELIDONIUM MAJUS 3X, CHOLESTERINUM 30C, ARSENICUM ALBUM 1M, BELLADONNA 1M, LYCOPODIUM CLAVATUM 1M, TARENTULA HISPANA 10M
INDICATIONS:
For the temporary relief of anger and rage.
WARNINGS:
If pregnant or breast-feeding, ask a health care professional before using.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in a cool dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Take as directed in the Restart Program or as follows: Put 5 drops under the tongue 2x daily as needed.
Consult a physician for use in children under 12 years of age.
INDICATIONS:
For the temporary relief of anger and rage.
INACTIVE INGREDIENTS:
25% Alcohol, Demineralized water.
QUESTIONS:
MFG FOR EVOLUTION,
269 S. BEVERLY DR., 110
BEVERLY HILLS, CA 90212
www.evo-inc.com
PACKAGE LABEL DISPLAY:
HOMEOPATHIC
Liver Align
The Restart Program
5
evolution
HOLISTIC HEALTH CARE
1 fl oz (30ml)