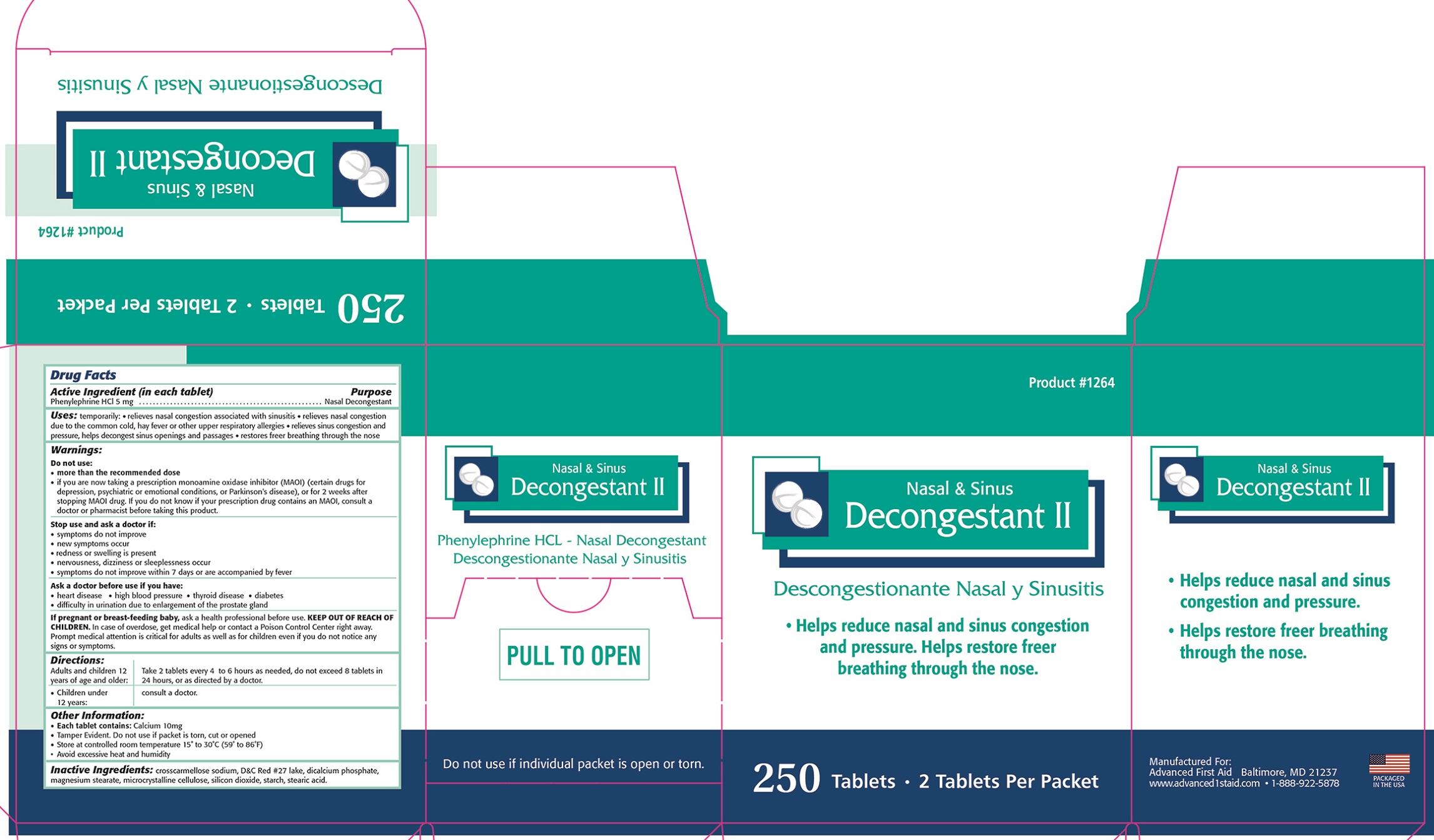
Uses: temporarily: • relieves nasal congestion associated with sinusitis •relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies • relieves sinus congestion and pressure, helps decongest sinus openings and passages • restores freer breathing through the nose
Warnings:
Do not use: • more than the recommended dose
• if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping MAOI drug. If you do not know if your prescription drug contains an MAOI, consult a doctor or pharmacist before taking this product.
Stop use and ask a doctor if:
• symptoms do not improve
• new symptoms occur
• redness or swelling is present
• nervousness, dizziness or sleeplessness occur
• symptoms do not improve within 7 days or are accompanied by fever
Ask a doctor before use if you have: • heart disease • high blood pressure • thyroid disease • diabetes • difficulty in urination due to enlargement of the prostate gland
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions:
Adults and children 12 years of age and older: Take 2 tablets every 4 to 6 hours or as needed, do not exceed 8 tablets in 24 hours, or as directed by a doctor.
Children under 12 years: Consult a doctor.