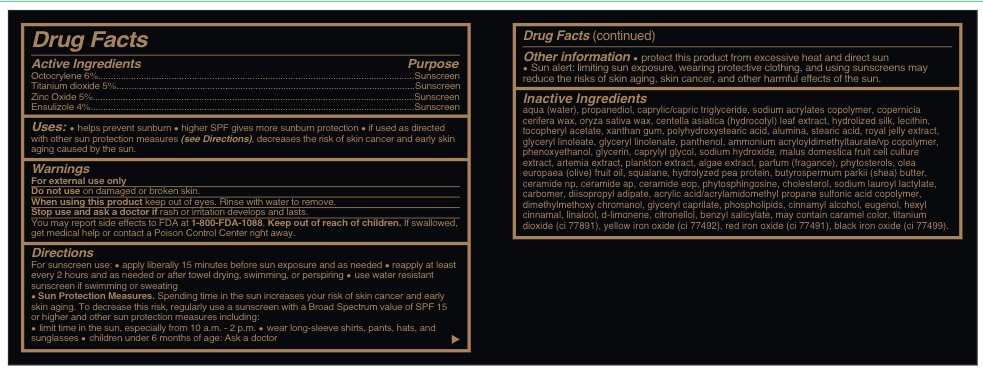
Active Ingredients......sunscreen
Octocrylene 6%.........Sunscreen
Titanium dioxide 5%.........Sunscreen
Zinc oxide 5%.........Sunscreen
Ensulizole 4%..........Sunscreen
Inactive Ingredients
AQUA (WATER), PROPANEDIOL, CAPRYLIC/CAPRIC TRIGLYCERIDE, SODIUM ACRYLATES COPOLYMER, COPERNICIA CERIFERA WAX, ORYZA SATIVA WAX, CENTELLA ASIATICA (HYDROCOTYL) LEAF EXTRACT, HYDROLIZED SILK, LECITHIN, TOCOPHERYL ACETATE, XANTHAN GUM, POLYHYDROXYSTEARIC ACID, ALUMINA, STEARIC ACID, ROYAL JELLY EXTRACT, GLYCERYL LINOLEATE, GLYCERYL LINOLENATE, PANTHENOL, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, PHENOXYETHANOL, GLYCERIN, CAPRYLYL GLYCOL, SODIUM HYDROXIDE, MALUS DOMESTICA FRUIT CELL CULTURE EXTRACT, ARTEMIA EXTRACT, PLANKTON EXTRACT, ALGAE EXTRACT, PARFUM (FRAGANCE), PHYTOSTEROLS, OLEA EUROPAEA (OLIVE) FRUIT OIL, SQUALANE, HYDROLYZED PEA PROTEIN, BUTYROSPERMUM PARKII (SHEA) BUTTER, CERAMIDE NP, CERAMIDE AP, CERAMIDE EOP, PHYTOSPHINGOSINE, CHOLESTEROL, SODIUM LAUROYL LACTYLATE, CARBOMER, DIISOPROPYL ADIPATE, ACRYLIC ACID/ACRYLAMIDOMETHYL PROPANE SULFONIC ACID COPOLYMER, DIMETHYLMETHOXY CHROMANOL, GLYCERYL CAPRILATE, PHOSPHOLIPIDS, CARAMEL, CI 77891, CI 77492, CI 77491 , CI 77499 , CINNAMYL ALCOHOL, EUGENOL, HEXYL CINNAMAL, LINALOOL, d-LIMONENE, CITRONELLOL, BENZYL SALICYLATE.
Uses
helps prevent sunburn * higher SPF gives more sunburn protection * if used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash or irratation develos and lasts.
You may report effects to FDA at 1-800-FDA-1088.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
For suncreen use: *apply liberally 15 minutes before sun exposure and as needed * reapply at least every 2 hours and as needed or after towel drying, swimming, or perspiring. *. use water resistant sunscreen if swimming or sweating * Sun Protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum value of SPF 15 or higher and other sun protection measures including:
* limit time in th esun, especially from 10 a.m. - 2 p.m.
* wear long-sleeve shirts, pants, hats and sunglasses
*children under 6 months of age: Ask a doctor
Other information
Other information * protect this product from excessive heat and direct sun. * Sun alert: limiting sun exposure, wearing protective clothing, and using sunscreen may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun.
Description
RADIANCE SUPERBE DAY CREAM
BROAD SPECTRUM SPF 35
OIL-FREE SUNSCREEN
A latest generation Triple Age Management Sunscreen with an immediate ultra-sheer complexion perfecting finish availalbe in 6 ultra-sheer shades + translucent, leaving skin with a radiant, healthy glow. Oil free, for all skin types, including sensitive skin.
Haute Custom Beauty SLU
Passeig de Gracia 41
08047 Barcelona
Haute Custom Beauty INC
Austin, TX 78703 USA
PRODUCT OF SPAIN
www.hautecustombeauty.com
Airplane image OK
Gluten Free
No: parabens, sulphates, mineral oil, silicons, phthalates, PEGS, methysilothazzolinone, petroleum derivatives