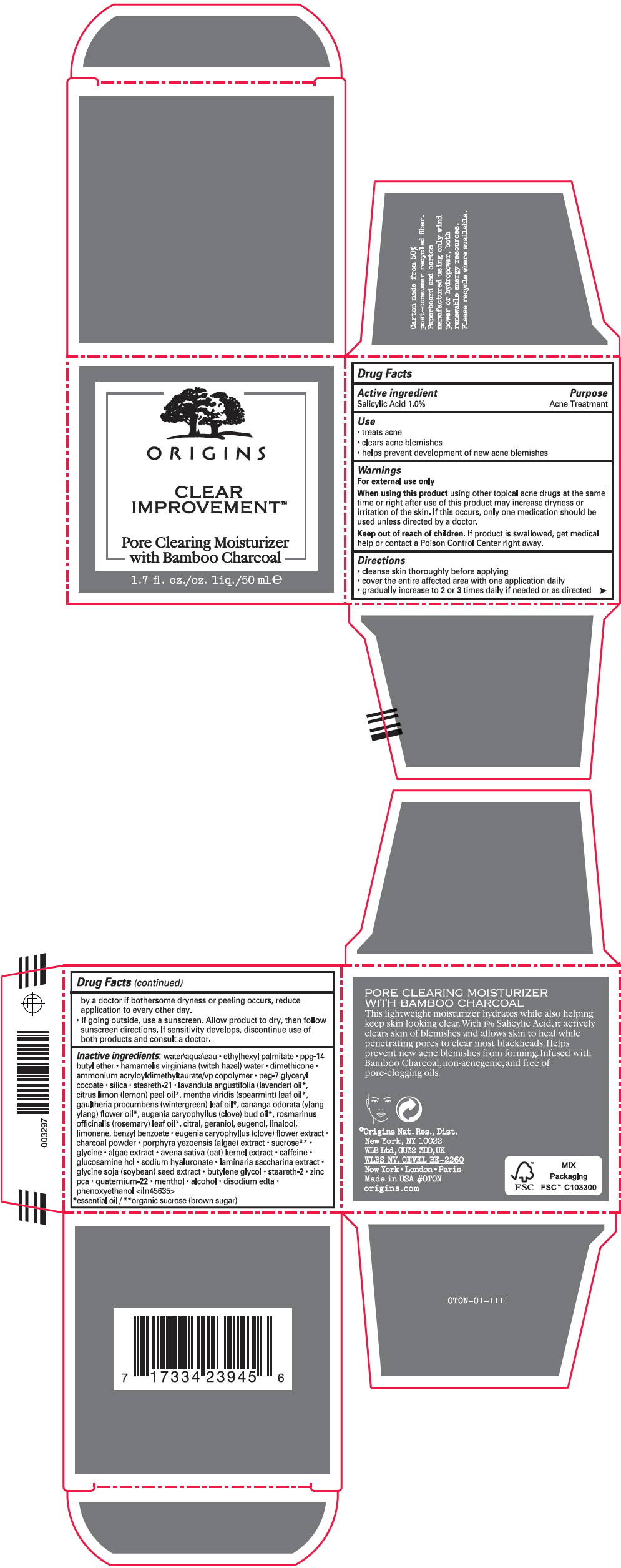
Warnings
For external use only
Directions
- cleanse skin thoroughly before applying
- cover the entire affected area with one application daily
- gradually increase to 2 or 3 times daily if needed or as directed by a doctor if bothersome dryness or peeling occurs, reduce application to every other day.
- If going outside, use a sunscreen. Allow product to dry, then follow sunscreen directions. If sensitivity develops, discontinue use of both products and consult a doctor.
Inactive ingredients
water\aqua\eau • ethylhexyl palmitate • ppg-14 butyl ether • hamamelis virginiana (witch hazel) water • dimethicone • ammonium acryloyldimethyltaurate/vp copolymer • peg-7 glyceryl cocoate • silica • steareth-21 • lavandula angustifolia (lavender) oil 1, citrus limon (lemon) peel oil 1, mentha viridis (spearmint) leaf oil 1, gaultheria procumbens (wintergreen) leaf oil 1, cananga odorata (ylang ylang) flower oil 1, eugenia caryophyllus (clove) bud oil 1, rosmarinus officinalis (rosemary) leaf oil 1, citral, geraniol, eugenol, linalool, limonene, benzyl benzoate • eugenia caryophyllus (clove) flower extract • charcoal powder • porphyra yezoensis (algae) extract • sucrose 2 • glycine • algae extract • avena sativa (oat) kernel extract • caffeine • glucosamine hcl • sodium hyaluronate • laminaria saccharina extract • glycine soja (soybean) seed extract • butylene glycol • steareth-2 • zinc pca • quaternium-22 • menthol • alcohol • disodium edta • phenoxyethanol <iln45635>