BE GONE SINUS CONGESTION TM- oyster shell calcium carbonate, crude - goldenseal - potassium dichromate pellet
Washington Homeopathic Products
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS
CALC CARB 6C
HYDRASTIS 6C
KALI BIC 6C
USES
To relieve the symptoms of congested sinuses and headaches relating to congested sinuses.
KEEP OUT OF REACH OF CHILDREN
As with all medications, keep out of reach of children.
INDICATIONS
Indications:
CALC CARB - OVERWORK
HYDRASTIS - SINUSES
KALI BIC - SINUSES
STOP USE AND ASK DOCTOR
If symptoms persist or recur, discontinue use. If pregnant or nursing a baby, consult a licensed practitioner before using this product.
DIRECTIONS
Adults 2 pellets every 3 hours for 2 days. Then 2 pellets morning and night for 2 weeks.
Children: 1 pellet. Repeat as necessary.
INACTIVE INGREDIENTS
Sucrose/Lactose
PRINCIPAL DISPLAY PANEL
 Be g
oneTM Sinus Congesion label
Be g
oneTM Sinus Congesion label
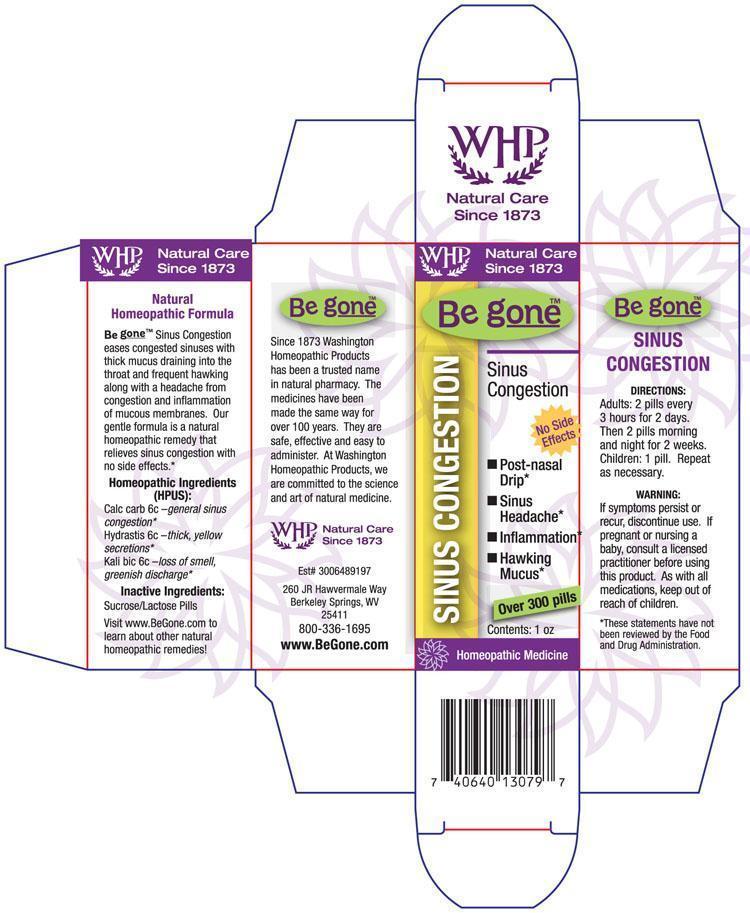 Be g
oneTM Sinus Congestion box
Be g
oneTM Sinus Congestion box