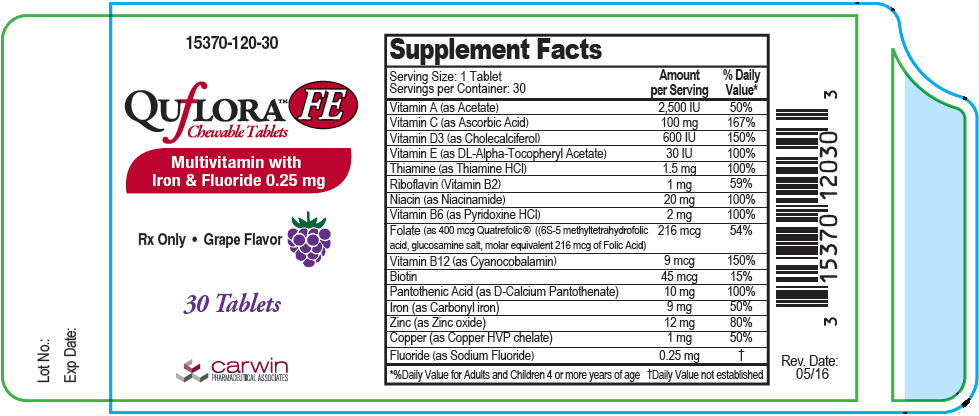
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Tablet Servings per Container: 30 | ||
| Amount per Serving | % Daily Value* | |
| Vitamin A (as Acetate) | 2500 IU | 50% |
| Vitamin C (as Ascorbic Acid) | 100 mg | 167% |
| Vitamin D3 ( as Cholecalciferol) | 600 IU | 150% |
| Vitamin E (as DL-Alpha Tocopheryl Acetate) | 30 IU | 100% |
| Thiamine (as Thiamine HCl) | 1.5 mg | 100% |
| Riboflavin (Vitamin B2) | 1 mg | 59% |
| Niacin (as Niacinamide) | 20 mg | 100% |
| Vitamin B6 (as Pyridoxine HCl) | 2 mg | 100% |
| Folate (as 400 mcg Quatrefolic® ((6S-5 methyltetrahydrofolic acid, glucosamine salt, molar equivalent 216 mcg of Folic Acid) | 216 mcg | 54% |
| Vitamin B12 (as Cyanocobalamin) | 9 mcg | 150% |
| Biotin | 45 mcg | 15% |
| Pantothenic Acid (as D-Calcium Pantothenate) | 10 mg | 100% |
| Iron (as Carbonyl iron) | 9 mg | 50% |
| Zinc (as Zinc oxide) | 12 mg | 80% |
| Copper (as Copper HVP chelate) | 1 mg | 50% |
| Fluoride (as Sodium Fluoride) | 0.25 mg | † |
Other Ingredients: Citric acid, fumed silica, magnesium stearate, microcrystalline cellulose, natural grape flavor, silicon dioxide, sorbitol, stearic acid, sucralose and xylitol.
INDICATIONS AND USAGE
Quflora™ FE Chewable Tablets Multivitamin with Iron and Fluoride 0.25mg is a prescription dietary fluoride supplement providing essential vitamins and minerals.
CONTRAINDICATIONS
Quflora™ FE Chewable Tablets Multivitamin with Iron and Fluoride 0.25 mg should not be used by patients with a known history of hypersensitivity to any of the listed ingredients.
PRECAUTIONS
General
The suggested dose should not be exceeded, since dental fluorosis may result from continued ingestion of large amounts of fluoride. Do not eat or drink dairy products within one hour of fluoride administration. Incompatibility of fluoride with dairy foods has been reported due to formation of calcium fluoride which is poorly absorbed. Your healthcare practitioner can prescribe the correct dosage.
Folic Acid
Folic Acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS
Allergic rash and other idiosyncrasies have been rarely reported. Call your doctor for medical advice about side effects. You may report side effects or obtain product information by calling Carwin Pharmaceutical Associates, LLC at 1-844-700-5011.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
DOSAGE AND ADMINISTRATION
One tablet daily or as prescribed by your healthcare practitioner. This product should be chewed and is not recommended for children under age 4.
DESCRIPTION
Quflora™ FE Chewable Tablets is a gray with yellowish tint, round tablet imprinted "120".
HOW SUPPLIED
Quflora™ FE Chewable Tablets are available in child-resistant bottles of 30 (Product Code: 15370-120-30).
Rx Only
STORAGE
Store at controlled room temperature 20° C to 25° C (68° F to 77° F), excursions permitted between 15° C and 30° C (59° F and 86° F). Protect from light, moisture and excessive heat.
THIS PRODUCT CONTAINS IRON.
KEEP THIS PRODUCT OUT OF REACH OF CHILDREN.
TAMPER EVIDENT: Do not use if seal is broken or missing.
Quatrefolic® is a registered trademark of Gnosis, SpA. Covered by one or more claims of U.S. Patent # 7,947,662 CAS# 1181972-37-1