TOBI PODHALER- tobramycin capsule
Mylan Specialty L.P.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TOBI PODHALER safely and effectively. See full prescribing information for TOBI PODHALER.
TOBI® PODHALER® (tobramycin inhalation powder), for oral inhalation use Initial U.S. Approval: 1975 INDICATIONS AND USAGETOBI Podhaler is an aminoglycoside antibacterial indicated for the management of cystic fibrosis patients with Pseudomonas aeruginosa. Safety and efficacy have not been demonstrated in patients under the age of 6 years, patients with forced expiratory volume in 1 second (FEV1) <25% or >80%, or patients colonized with Burkholderia cepacia (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSInhalation powder: 28 mg in a capsule (3) CONTRAINDICATIONSKnown hypersensitivity to any aminoglycoside (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions (≥10 % of TOBI Podhaler and TOBI patients in primary safety population) are cough, lung disorder, productive cough, dyspnea, pyrexia, oropharyngeal pain, dysphonia, hemoptysis, and headache (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSConcurrent and/or sequential use of TOBI Podhaler with other drugs with neurotoxic, nephrotoxic, or ototoxic potential should be avoided (7) See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 7/2020 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
TOBI Podhaler is indicated for the management of cystic fibrosis patients with Pseudomonas aeruginosa.
Safety and efficacy have not been demonstrated in patients under the age of 6 years, patients with forced expiratory volume in 1 second (FEV1) <25% or >80% predicted, or patients colonized with Burkholderia cepacia [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
DO NOT SWALLOW TOBI PODHALER CAPSULES
FOR USE WITH THE PODHALER DEVICE ONLY
FOR ORAL INHALATION ONLY
TOBI Podhaler capsules must not be swallowed as the intended effects in the lungs will not be obtained. The contents of TOBI Podhaler capsules are only for oral inhalation and should only be used with the Podhaler device.
The recommended dosage of TOBI Podhaler for both adults and pediatric patients 6 years of age and older is the inhalation of the contents of four 28 mg TOBI Podhaler capsules twice-daily for 28 days using the Podhaler device.
Refer to the Instructions For Use (IFU) for full administration information.
Dosage is not adjusted by weight. Each dose of four capsules should be taken as close to 12 hours apart as possible; each dose should not be taken less than 6 hours apart.
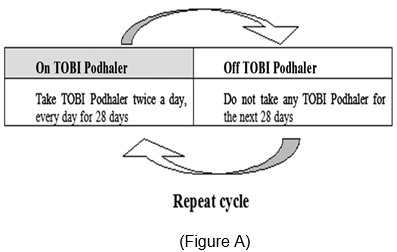
TOBI Podhaler is administered twice-daily in alternating periods of 28 days. After 28 days of therapy, patients should stop TOBI Podhaler therapy for the next 28 days, and then resume therapy for the next 28-day on and 28-day off cycle.
TOBI Podhaler capsules should always be stored in the blister and each capsule should only be removed IMMEDIATELY BEFORE USE.
For patients taking several different inhaled medications and/or performing chest physiotherapy, the order of therapies should follow the physician’s recommendation. It is recommended that TOBI Podhaler is taken last.
3 DOSAGE FORMS AND STRENGTHS
Inhalation powder:
28 mg: clear, colorless hypromellose capsule with “NVR AVCI” in blue radial imprint on one part of the capsule and the Novartis logo “ ” in blue radial imprint on the other part of the capsule.
” in blue radial imprint on the other part of the capsule.
4 CONTRAINDICATIONS
TOBI Podhaler is contraindicated in patients with a known hypersensitivity to any aminoglycoside.
5 WARNINGS AND PRECAUTIONS
5.1 Bronchospasm
Bronchospasm has been reported with inhalation of TOBI Podhaler [see Adverse Reactions (6.1)]. Bronchospasm should be treated as medically appropriate.
5.2 Ototoxicity
Caution should be exercised when prescribing TOBI Podhaler to patients with known or suspected auditory or vestibular dysfunction.
Ototoxicity, as measured by complaints of hearing loss or tinnitus, was reported by patients in the TOBI Podhaler clinical studies [see Adverse Reactions (6.1)]. Tinnitus may be a sentinel symptom of ototoxicity, and therefore the onset of this symptom warrants caution. Ototoxicity, manifested as both auditory (hearing loss) and vestibular toxicity, has been reported with parenteral aminoglycosides. Vestibular toxicity may be manifested by vertigo, ataxia or dizziness.
5.3 Nephrotoxicity
Caution should be exercised when prescribing TOBI Podhaler to patients with known or suspected renal dysfunction.
Nephrotoxicity was not observed during TOBI Podhaler clinical studies but has been associated with aminoglycosides as a class.
5.4 Neuromuscular Disorders
Caution should be exercised when prescribing TOBI Podhaler to patients with known or suspected neuromuscular dysfunction.
TOBI Podhaler should be used cautiously in patients with neuromuscular disorders, such as myasthenia gravis or Parkinson’s disease, since aminoglycosides may aggravate muscle weakness because of a potential curare-like effect on neuromuscular function.
5.5 Embryo-Fetal Toxicity
Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides cross the placenta, and streptomycin has been associated with several reports of total, irreversible, bilateral congenital deafness in pediatric patients exposed in utero. However, systemic absorption of tobramycin following inhaled administration is expected to be minimal [see Clinical Pharmacology (12.3)]. Patients who use TOBI Podhaler during pregnancy, or become pregnant while taking TOBI Podhaler should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
TOBI Podhaler has been evaluated for safety in 425 cystic fibrosis patients exposed to at least one dose of TOBI Podhaler, including 273 patients who were exposed across three cycles (6 months) of treatment. Each cycle consisted of 28 days on-treatment (with 112 mg administered twice-daily) and 28 days off-treatment. Patients with serum creatinine ≥2 mg/dL and blood urea nitrogen (BUN) ≥40 mg/dL were excluded from clinical studies. There were 218 males and 207 females in this population, and reflecting the cystic fibrosis population in the U.S., the vast majority of patients were Caucasian. There were 221 patients ≥20 years old, 121 patients ≥13 to <20 years old, and 83 patients ≥6 to <13 years old. There were 239 patients with screening FEV1 % predicted ≥50%, 156 patients with screening FEV1 % predicted <50%, and 30 patients with missing FEV1 % predicted.
The primary safety population reflects patients from Study 1, an open-label study comparing TOBI Podhaler with TOBI (tobramycin inhalation solution, USP) over three cycles of 4 weeks on treatment followed by 4 weeks off treatment. Randomization, in a planned 3:2 ratio, resulted in 308 patients treated with TOBI Podhaler and 209 patients treated with TOBI. For both the TOBI Podhaler and TOBI groups, mean exposure to medication for each cycle was 28 to 29 days. The mean age for both arms was between 25 and 26 years old. The mean baseline FEV1 % predicted for both arms was 53%.
Table 1 displays adverse drug reactions reported by at least 2% of TOBI Podhaler patients in Study 1, inclusive of all cycles (on and off treatment). Adverse drug reactions are listed according to MedDRA system organ class and sorted within system organ class group in descending order of frequency.
|
||
|
Primary System Organ Class |
TOBI Podhaler |
TOBI |
|
Respiratory, thoracic, and mediastinal disorders | ||
|
Cough |
48.4 |
31.1 |
|
Lung disorder* |
33.8 |
30.1 |
|
Productive cough |
18.2 |
19.6 |
|
Dyspnea |
15.6 |
12.4 |
|
Oropharyngeal pain |
14.0 |
10.5 |
|
Dysphonia |
13.6 |
3.8 |
|
Hemoptysis |
13.0 |
12.4 |
|
Nasal congestion |
8.1 |
7.2 |
|
Rales |
7.1 |
6.2 |
|
Wheezing |
6.8 |
6.2 |
|
Chest discomfort |
6.5 |
2.9 |
|
Throat irritation |
4.5 |
1.9 |
|
Gastrointestinal disorders | ||
|
Nausea |
7.5 |
9.6 |
|
Vomiting |
6.2 |
5.7 |
|
Diarrhea |
4.2 |
1.9 |
|
Dysgeusia |
3.9 |
0.5 |
|
Infections and infestations | ||
|
Upper respiratory tract infection |
6.8 |
8.6 |
|
Investigations | ||
|
Pulmonary function test decreased |
6.8 |
8.1 |
|
Forced expiratory volume decreased |
3.9 |
1.0 |
|
Blood glucose increased |
2.9 |
0.5 |
|
Vascular disorders | ||
|
Epistaxis |
2.6 |
1.9 |
|
Nervous system disorders | ||
|
Headache |
11.4 |
12.0 |
|
General disorders and administration site conditions | ||
|
Pyrexia |
15.6 |
12.4 |
|
Musculoskeletal and connective tissue disorders | ||
|
Musculoskeletal chest pain |
4.5 |
4.8 |
|
Skin and subcutaneous tissue disorders | ||
|
Rash |
2.3 |
2.4 |
Adverse drug reactions that occurred in <2% of patients treated with TOBI Podhaler in Study 1 were: bronchospasm (TOBI Podhaler 1.6%, TOBI 0.5%); deafness including deafness unilateral (reported as mild to moderate hearing loss or increased hearing loss) (TOBI Podhaler 1.0%, TOBI 0.5%); and tinnitus (TOBI Podhaler 1.9%, TOBI 2.4%).
Discontinuations in Study 1 were higher in the TOBI Podhaler arm compared to TOBI (27% TOBI Podhaler versus 18% TOBI). This was driven primarily by discontinuations due to adverse events (14% TOBI Podhaler versus 8% TOBI). Higher rates of discontinuation were seen in subjects ≥20 years old and those with baseline FEV1 % predicted <50%.
Respiratory related hospitalizations occurred in 24% of the patients in the TOBI Podhaler arm and 22% of the patients in the TOBI arm. There was an increased new usage of antipseudomonal medication in the TOBI Podhaler arm (65% TOBI Podhaler versus 55% TOBI). This included oral antibiotics in 55% of TOBI Podhaler patients and 40% of TOBI patients and intravenous antibiotics in 35% of TOBI Podhaler patients and 33% of TOBI patients. Median time to first antipseudomonal usage was 89 days in the TOBI Podhaler arm and 112 days in the TOBI arm.
The supportive safety population reflects patients from two studies: Study 2, a double-blind, placebo-controlled design for the first treatment cycle, followed by all patients receiving TOBI Podhaler (replaced placebo) for two additional cycles, and Study 3, a double-blind, placebo-controlled trial for one treatment cycle only. Placebo in these studies was inhaled powder without the active ingredient, tobramycin. The patient population for these studies was much younger than in Study 1 (mean age 13 years old).
Adverse drug reactions reported more frequently by TOBI Podhaler patients in the placebo-controlled cycle (Cycle 1) of Study 2, which included 46 TOBI Podhaler and 49 placebo patients, were:
Respiratory, thoracic, and mediastinal disorders
Pharyngolaryngeal pain (TOBI Podhaler 10.9%, placebo 0%); dysphonia (TOBI Podhaler 4.3%, placebo 0%)
Gastrointestinal disorders
Dysgeusia (TOBI Podhaler 6.5%, placebo 2.0%)
Adverse drug reactions reported more frequently by TOBI Podhaler patients in Study 3, which included 30 TOBI Podhaler and 32 placebo patients, were:
Respiratory, thoracic, and mediastinal disorders
Cough (TOBI Podhaler 10%, placebo 0%)
Ear and labyrinth disorders
Hypoacusis (TOBI Podhaler 10%, placebo 6.3%)
Audiometric Assessment
In Study 1, audiology testing was performed in a subset of approximately 25% of TOBI Podhaler (n=78) and TOBI (n=45) patients. Using the criteria for either ear of ≥10 dB loss at two consecutive frequencies, ≥20 dB loss at any frequency, or loss of response at three consecutive frequencies where responses were previously obtained, five TOBI Podhaler patients and three TOBI patients were judged to have ototoxicity, a ratio similar to the planned 3:2 randomization for this study.
Audiology testing was also performed in a subset of patients in both Study 2 (n=13 from the TOBI Podhaler group and n=9 from the placebo group) and Study 3 (n=14 from the TOBI Podhaler group and n=11 from the placebo group). In Study 2, no patients reported hearing complaints but two TOBI Podhaler patients met the criteria for ototoxicity. In Study 3, three TOBI Podhaler and two placebo patients had reports of ‘hypoacusis.’ One TOBI Podhaler and two placebo patients met the criteria for ototoxicity. In some patients, ototoxicity was transient or may have been related to a conductive defect.
Cough
Cough is a common symptom in cystic fibrosis, reported in 42% of the patients in Study 1 at baseline. Cough was the most frequently reported adverse event in Study 1 and was more common in the TOBI Podhaler arm (48% TOBI Podhaler versus 31 % TOBI). There was a higher rate of cough adverse event reporting during the first week of active treatment with TOBI Podhaler (i.e., the first week of Cycle 1). The time to first cough event in the TOBI Podhaler and TOBI groups were similar thereafter. In some patients, cough resulted in discontinuation of TOBI Podhaler treatment. Sixteen patients (5%) receiving treatment with TOBI Podhaler discontinued study treatment due to cough events compared with 2 (1%) in the TOBI treatment group. Children and adolescents coughed more than adults when treated with TOBI Podhaler, yet the adults were more likely to discontinue: of the 16 patients on TOBI Podhaler in Study 1 who discontinued treatment due to cough events, 14 were ≥20 years of age, one patient was between the ages of 13 and <20, and one was between the ages of 6 and <13. The rates of bronchospasm (as measured by ≥20% decrease in FEV1 % predicted post-dose) were approximately 5% in both treatment groups, and none of these patients experienced concomitant cough.
In Study 2, cough was the most commonly reported adverse event during the first cycle of treatment (the double blind period of treatment) and occurred more frequently in placebo-treated patients (26.5%) than patients treated with TOBI Podhaler (13%). Similar percentages of patients in both treatment groups reported cough as a baseline symptom. In Study 3, cough events were reported by three patients in the TOBI Podhaler group (10%) and none in the placebo group (0%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of TOBI Podhaler. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Respiratory, thoracic, and mediastinal disorders
Aphonia, Sputum discolored
General disorders and administration site conditions
Malaise
7 DRUG INTERACTIONS
No clinical drug interaction studies have been performed with TOBI Podhaler. In clinical studies, patients receiving TOBI Podhaler continued to take dornase alfa, bronchodilators, inhaled corticosteroids, and macrolides. No clinical signs of drug interactions with these medicines were identified.
Concurrent and/or sequential use of TOBI Podhaler with other drugs with neurotoxic, nephrotoxic, or ototoxic potential should be avoided.
Some diuretics can enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue. TOBI Podhaler should not be administered concomitantly with ethacrynic acid, furosemide, urea, or intravenous mannitol. The interaction between inhaled mannitol and TOBI Podhaler has not been evaluated.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Aminoglycosides can cause fetal harm. Published literature reports that use of streptomycin, an aminoglycoside, can cause total, irreversible, bilateral congenital deafness when administered to a pregnant woman [Warnings and Precautions (5.5)]. Although there are no available data on TOBI Podhaler use in pregnant women to inform a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes, systemic absorption of tobramycin following inhaled administration is expected to be minimal [see Clinical Pharmacology (12.3)]. There are risks to the mother associated with cystic fibrosis in pregnancy (see Clinical Considerations). In animal reproduction studies with subcutaneous administration of tobramycin in pregnant rats and rabbits during organogenesis there were no adverse developmental outcomes; however, ototoxicity was not evaluated in the offspring from these studies (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
No reproduction toxicology studies have been conducted with TOBI Podhaler. However, subcutaneous administration of tobramycin at doses of up to 100 (rat) or 20 (rabbit) mg/kg/day during organogenesis was not associated with adverse developmental outcomes. Doses of tobramycin ≥40 mg/kg/day were severely maternally toxic to rabbits and precluded the evaluation of adverse developmental outcomes. Ototoxicity was not evaluated in offspring during non-clinical reproductive toxicity studies with tobramycin.
8.2 Lactation
Risk Summary
There are no data on the presence of tobramycin following administration of TOBI Podhaler in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. Limited published data on other formulations of tobramycin in lactating women indicate that tobramycin is present in human milk. However, systemic absorption of tobramycin following inhaled administration is expected to be minimal [see Clinical Pharmacology (12.3)]. Tobramycin may cause alteration in the intestinal flora of the breastfeeding infant (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TOBI Podhaler and any potential adverse effects on the breastfed infant from TOBI Podhaler or from the underlying maternal condition.
8.4 Pediatric Use
Patients 6 years and older were included in the Phase 3 studies with TOBI Podhaler; 206 patients below 20 years of age received TOBI Podhaler. No dosage adjustments are needed based on age. The overall pattern of adverse events in pediatric patients was similar to the adults. Dysgeusia (taste disturbance) was more commonly reported in younger patients six to 19 years of age than in patients 20 years and older, 7.4% versus 2.7%, respectively. Safety and effectiveness in pediatric patients below the age of 6 years have not been established.
8.5 Geriatric Use
Clinical studies of TOBI Podhaler did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Tobramycin is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function [see Warnings and Precautions (5.2, 5.5)].
8.6 Renal Impairment
Tobramycin is primarily excreted unchanged in the urine and renal function is expected to affect the exposure to tobramycin. The risk of adverse reactions to this drug may be greater in patients with impaired renal function. Patients with serum creatinine ≥2 mg/dL and blood urea nitrogen (BUN) ≥40 mg/dL have not been included in clinical studies and there are no data in this population to support a recommendation regarding dose adjustment with TOBI Podhaler [see Warnings and Precautions (5.2, 5.5)].
10 OVERDOSAGE
The maximum tolerated daily dose of TOBI Podhaler has not been established.
In the event of accidental oral ingestion of TOBI Podhaler capsules, systemic toxicity is unlikely as tobramycin is poorly absorbed. Tobramycin serum concentrations may be helpful in monitoring overdosage.
Acute toxicity should be treated with immediate withdrawal of TOBI Podhaler, and baseline tests of renal function should be undertaken.
Hemodialysis may be helpful in removing tobramycin from the body.
In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment. In the case of any overdosage, the possibility of drug interactions with alterations in drug disposition should be considered.
11 DESCRIPTION
TOBI Podhaler consists of a dry powder formulation of tobramycin for oral inhalation only with the Podhaler device. The inhalation powder is filled into clear, colorless hypromellose capsules.
Each clear, colorless hypromellose capsule contains a spray dried powder of 28 mg of tobramycin active ingredient with 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), calcium chloride, and sulfuric acid (for pH adjustment).
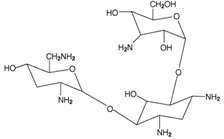
The active component of TOBI Podhaler is tobramycin. Tobramycin is an aminoglycoside antibiotic. Its chemical name is O-3-amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribo-hexopyranosyl-(1→6)]-2-deoxy-L-streptamine; its structural formula is:
Tobramycin has a molecular weight of 467.52, and its empirical formula is C18H37N5O9. Tobramycin is a white to almost white powder; visually free from any foreign contaminants. Tobramycin is freely soluble in water, very slightly soluble in ethanol, and practically insoluble in chloroform and ether.
The Podhaler device is a plastic device used to inhale the dry powder contained in the TOBI Podhaler capsule. Under standardized in vitro testing at a fixed flow rate of 60 L/min and volume of 2 L for 2 seconds, the Podhaler device has a target delivered dose of 102 mg of tobramycin from the mouthpiece (4 capsules per dose). Peak inspiratory flow rate and inhaled volumes were explored in 96 cystic fibrosis patients aged 6 years and older. Older patients with significant disease progression and associated decreases in forced expiratory volume (FEV1) and younger patients with inhaled volumes <1 L were able to generate inspiratory flow rates and volumes required to receive their medication when following the instructions for use. However, no pediatric patients aged 6 to 10 years with FEV1 less than 40% predicted were evaluated.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tobramycin is an aminoglycoside antibacterial [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Absorption
TOBI Podhaler contains tobramycin, a cationic polar molecule that does not readily cross epithelial membranes. TOBI Podhaler is specifically formulated for administration by oral inhalation. The systemic exposure to tobramycin after inhalation of TOBI Podhaler is expected to result from pulmonary absorption of the dose fraction delivered to the lungs as tobramycin and is not absorbed to any appreciable extent when administered via the oral route.
Serum Concentrations
After inhalation of a 112 mg single dose (4 times 28 mg capsules) of TOBI Podhaler in cystic fibrosis patients, the maximum serum concentration (Cmax) of tobramycin was 1.02 ± 0.53 mcg/mL (mean ± SD) and the median time to reach the peak concentration (Tmax) was 1 hour. In comparison, after inhalation of a single 300 mg dose of TOBI, Cmax was 1.04 ± 0.58 mcg/mL and median Tmax was 1 hour. The extent of systemic exposure (AUC0-12) was also similar: 4.6 ± 2.0 mcg∙h/mL following the 112 mg TOBI Podhaler dose and 4.8 ± 2.5 mcg∙h/mL following the 300 mg TOBI dose. At the end of a 4-week dosing cycle of TOBI Podhaler (112 mg twice-daily), the maximum serum concentration of tobramycin 1 hour after dosing ranged from 1.48 ± 0.69 mcg/mL to 1.99 ± 0.59 mcg/mL (mean ± SD).
Sputum Concentrations
After inhalation of a 112 mg single dose (4 times 28 mg capsules) of TOBI Podhaler in cystic fibrosis patients, sputum Cmax of tobramycin was 1048 ± 1080 mcg/g (mean ± SD). In comparison, after inhalation of a single 300 mg dose of TOBI, sputum Cmax was 737 ± 1028 mcg/g. The variability in pharmacokinetic parameters was higher in sputum as compared to serum.
Distribution
A population pharmacokinetic analysis for TOBI Podhaler in cystic fibrosis patients estimated the apparent volume of distribution of tobramycin in the central compartment to be 85.1 L for a typical cystic fibrosis (CF) patient.
Binding of tobramycin to serum proteins is negligible.
Elimination
Tobramycin is eliminated from the systemic circulation primarily by glomerular filtration of the unchanged compound. Systemically absorbed tobramycin following TOBI Podhaler administration is also expected to be eliminated principally by glomerular filtration.
The apparent terminal half-life of tobramycin in serum after inhalation of a 112 mg single dose of TOBI Podhaler was approximately 3 hours in cystic fibrosis patients and consistent with the half-life of tobramycin after TOBI inhalation.
A population pharmacokinetic analysis for TOBI Podhaler in cystic fibrosis patients aged 6 to 58 years estimated the apparent serum clearance of tobramycin to be 14.5 L/h. No clinically relevant covariates that were predictive of tobramycin clearance were identified from this analysis.
12.4 Microbiology
Mechanism of Action
Tobramycin is an aminoglycoside antibacterial produced by Streptomyces tenebrarius. It acts primarily by disrupting protein synthesis leading to altered cell membrane permeability, progressive disruption of the cell envelope, and eventual cell death.
Tobramycin has in vitro activity against Gram-negative bacteria including P. aeruginosa. It is bactericidal in vitro at peak concentrations equal to or slightly greater than the minimum inhibitory concentration (MIC).
Susceptibility Testing
Interpretive criteria for inhaled antibacterial products are not defined. The in vitro antimicrobial susceptibility test methods used to determine the susceptibility for parenteral tobramycin therapy can be used to monitor the susceptibility of P. aeruginosa isolated from cystic fibrosis patients. (1,2,3) The relationship between in vitro susceptibility test results and clinical outcome with TOBI Podhaler therapy is not clear. A single sputum sample from a cystic fibrosis patient may contain multiple morphotypes of P. aeruginosa and each morphotype may require a different concentration of tobramycin to inhibit its growth in vitro. Patients should be monitored for changes in tobramycin susceptibility.
Development of Resistance
In clinical studies, some increases from baseline to the end of the treatment period were observed in the tobramycin MIC for P. aeruginosa morphotypes. In general, a higher percentage of patients treated with TOBI Podhaler had increases in tobramycin MIC compared with placebo or patients treated with TOBI inhalation solution.
The clinical significance of changes in MICs for P. aeruginosa has not been clearly established in the treatment of cystic fibrosis patients.
Cross-Resistance
Some emerging resistance to aztreonam, ceftazidime, ciprofloxacin, imipenem, or meropenem were observed in the TOBI Podhaler clinical trials. As other anti-pseudomonal antibiotics were concomitantly utilized in many patients in the clinical trials, the association with TOBI Podhaler is not clear.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were not conducted with TOBI Podhaler. A 2-year rat inhalation toxicology study to assess carcinogenic potential of TOBI (tobramycin inhalation solution, USP) has been completed. Rats were exposed to TOBI for up to 1.5 hours per day for 95 weeks. Serum levels of tobramycin of up to 35 mcg/mL were measured in rats, in contrast to the maximum 1.99 ± 0.59 mcg/mL level observed in cystic fibrosis patients in TOBI Podhaler clinical trials. There was no drug-related increase in the incidence of any variety of tumor.
Additionally, tobramycin has been evaluated for genotoxicity in a battery of in vitro and in vivo tests. The Ames bacterial reversion test, conducted with 5 tester strains, failed to show a significant increase in revertants with or without metabolic activation in all strains. Tobramycin was negative in the mouse lymphoma forward mutation assay, did not induce chromosomal aberrations in Chinese hamster ovary cells, and was negative in the mouse micronucleus test.
Subcutaneous administration of up to 100 mg/kg of tobramycin did not affect mating behavior or cause impairment of fertility in male or female rats.
14 CLINICAL STUDIES
The Phase 3 clinical development program included two placebo-controlled studies (Studies 2 and 3) and one open-label study (Study 1), which randomized and dosed 157 and 517 patients, respectively, with a clinical diagnosis of cystic fibrosis, confirmed by quantitative pilocarpine iontophoresis sweat chloride test, well-characterized disease causing mutations in each CFTR gene, or abnormal nasal transepithelial potential difference characteristic of cystic fibrosis.
In the placebo-controlled studies, all patients were aged between 6 and 21 years old and had an FEV1 at screening within the range of 25% to 80% (inclusive) of predicted normal values for their age, sex, and height based upon Knudson criteria. In addition, all patients were infected with P. aeruginosa as demonstrated by a positive sputum or throat culture (or bronchoalveolar lavage) within 6 months prior to screening, and also in a sputum culture taken at the screening visit. Among the 76 patients treated with TOBI Podhaler, 37% were males and 63% were females. Thirty-six patients were between 6 and 12 years of age, and 40 patients were between 13 and 21 years of age. Patients had a mean baseline FEV1 of 56% of predicted normal value.
In both studies, >90% of patients received concomitant therapies for cystic fibrosis-related indications. The most frequently used other antibacterial drugs (any route of administration) were azithromycin, ciprofloxacin, and ceftazidime. Consistent with the population of cystic fibrosis patients, the most frequently used concomitant medications included oral pancreatic enzyme preparations, mucolytics (especially dornase alfa), and selective β2-adrenoreceptor agonists.
Study 2
Study 2 was a randomized, 3-cycle, 2-arm trial. Each cycle comprised of 28 days on treatment followed by 28 days off treatment. The first cycle was double-blind, placebo-controlled with eligible patients randomized 1:1 to TOBI Podhaler (4 times 28 mg capsules twice-daily) or placebo. Upon completion of the first cycle, patients who were randomized to the placebo treatment group received TOBI Podhaler for Cycles 2 and 3. The total treatment period was 24 weeks.
A total of 95 patients were randomized into Study 2 and received TOBI Podhaler (n=46) or placebo (n=49) in Cycle 1. All patients were less than 22 years of age (mean age 13.3 years) and had not received inhaled antipseudomonal antibiotics within four months prior to screening; 56% were female and 84% were Caucasian. This study was stopped early for demonstrated benefit and the primary analysis used the set of patients included in the interim analysis (n=79); 16 patients did not have data on the primary endpoint at that time. Of the 79 patients included in the interim analysis, 18 patients were excluded due to a failure to meet spirometry quality review criteria as determined by an external review panel. This resulted in a total of 61 patients, 29 in the TOBI Podhaler arm and 32 in the placebo arm, who were included in the primary analysis.
In the primary analysis, TOBI Podhaler significantly improved lung function compared with placebo as measured by the relative change in FEV1 % predicted from baseline to the end of Cycle 1 dosing. This analysis adjusted for the covariates of baseline FEV1 % predicted, age, and region, and imputed for missing data. Treatment with TOBI Podhaler and placebo resulted in relative increases in FEV1 % predicted of 12.54% and 0.09%, respectively (LS mean difference = 12.44%; 95% CI: 4.89, 20.00; p=0.002). Analysis of absolute changes in FEV1 % predicted showed LS means of 6.38% for TOBI Podhaler and -0.52% for placebo with a difference of 6.90% (95% CI: 2.40, 11.40). Improvements in lung function were achieved during the subsequent cycles of treatment with TOBI Podhaler, although the magnitude was reduced (Figure 1).
The percentage of patients using new antipseudomonal antibiotics in Cycle 1 was greater in the placebo treatment group (18.4%) compared with the TOBI Podhaler treatment group (13.1%). During the first cycle, 8.7% of TOBI Podhaler patients and 10.2% of placebo patients were treated with parenteral antipseudomonal antibiotics. In Cycle 1, two patients (4.4%) in the TOBI Podhaler treatment group required respiratory-related hospitalizations, compared with six patients (12.2%) in the placebo treatment group.
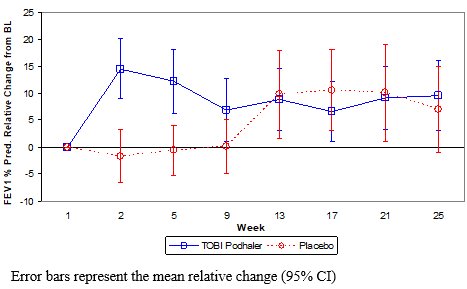
Figure 1 – Study 2: Mean Relative Change in FEV1 % Predicted from Baseline in Cycles 1 to 3 by Treatment Group
Study 3
Study 3 was a randomized, double-blind, placebo-controlled trial, similar in design to Study 2. Eligible patients were randomized 1:1 to receive TOBI Podhaler (4 times 28 mg capsules twice-daily) or placebo for one cycle (28 days on-treatment and 28 days off-treatment).
A total of 62 patients were randomized into Study 3 and allocated to TOBI Podhaler (n=32) or placebo (n=30). All patients were less than 22 years of age (mean age 12.9 years) and had not received inhaled antipseudomonal antibiotics within 4 months prior to screening; 64.5% were female and 98.4% were Caucasian.
In this study, the results were not statistically significant for the primary lung function endpoint when adjusting for the covariates of age (<13 years, ≥13 years) and FEV1 % predicted at screening (<50%, ≥50%) and imputing for missing data. Improvement in lung function for TOBI Podhaler compared with placebo was evaluated using the relative change in FEV1 % predicted from baseline to the end of Cycle 1 dosing. Treatment with TOBI Podhaler (8.19%) compared to placebo (2.27%) failed to achieve statistical significance in relative change in FEV1 % predicted (LS mean difference = 5.91%; 95% CI: -2.54, 14.37; p=0.167). Analyses of absolute changes in FEV1 % predicted showed LS means of 4.86% for TOBI Podhaler and 0.48% for placebo with a difference of 4.38% (95% CI:-0.17, 8.94).
Study 1
Study 1 was a randomized, open-label, active-controlled parallel arm trial. Eligible patients were randomized 3:2 to TOBI Podhaler (4 times 28 mg capsules twice-daily) or TOBI (300 mg/5 mL twice-daily). Treatment was administered for 28 days, followed by 28 days off therapy (one cycle) for three cycles. The total treatment period was 24 weeks. The time to administer a dose of TOBI Podhaler (10th to 90th percentiles) ranged from 2 to 7 minutes at the end of the dosing period for Cycle 1, and 2 to 6 minutes at the end of the dosing period for Cycle 3.
A total of 517 patients were randomized in Study 1 and received TOBI Podhaler (n=308) or TOBI (n=209). Patients were predominantly 20 years of age or older (mean age 25.6 years) with no inhaled antipseudomonal antibiotic use within 28 days prior to study drug administration; 45% were female and 91% were Caucasian.
The primary purpose of Study 1 was to evaluate safety. Interpretation of efficacy results in Study 1 is limited by several factors including open-label design, testing of multiple secondary endpoints, and missing values for the outcome of FEV1 % predicted. The number (%) of patients with missing values for FEV1 % predicted at Weeks 5 and 25 in the TOBI Podhaler treated group were 40 (13.0%) and 86 (27.9%) compared to 15 (7.2%) and 40 (19.1%) in the TOBI treated group. Using imputation of the missing data, the mean differences (TOBI Podhaler minus TOBI) in the percent relative change from baseline in FEV1 % predicted at Weeks 5 and 25 were -0.87 (95% CI: -3.80, 2.07) and 1.62 (95% CI: -0.90, 4.14), respectively.
15 REFERENCES
- 1.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically – Ninth Edition; Approved Standard. CLSI Document M7-A9. CLSI, 950 West Valley Rd., Suite 2500, Wayne, PA 19087, 2012.
- 2.
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard – 11th ed. CLSI document M02-A11. CLSI, 2012.
- 3.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; 22nd Informational Supplement. CLSI document M100-S22. CLSI, 2012
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
TOBI Podhaler contains aluminum blister-packaged 28 mg TOBI Podhaler (tobramycin inhalation powder) clear, colorless hypromellose capsules with “NVR AVCI” in blue radial imprint on one part of the capsule and the Novartis logo “ ” in blue radial imprint on the other part of the capsule, and Podhaler devices.
” in blue radial imprint on the other part of the capsule, and Podhaler devices.
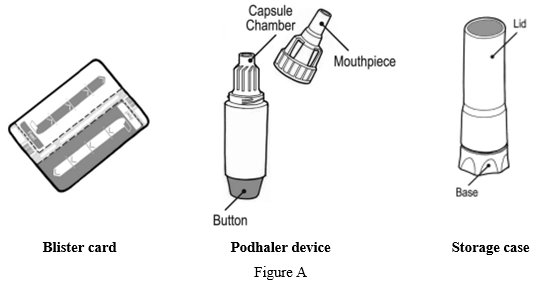
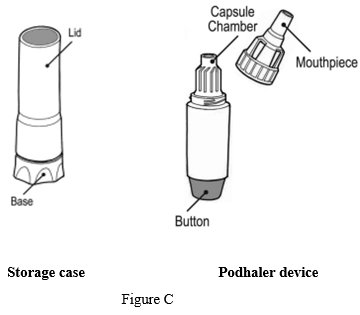
Each Podhaler device consists of the inhaler body, mouthpiece, capsule chamber and blue push button. The Podhaler device is provided in a case that protects the device during shipment, storage and its one week in-use period.
Unit dose (blister pack), Box of 224 capsules contains: NDC 49502-346-24
- 4 weekly packs, each containing:
- 56 capsules (7 blister cards of 8 capsules)
- 1 Podhaler device
- 1 reserve Podhaler device
Unit dose (blister pack), Box of 56 capsules (7-day pack) contains: NDC 49502-346-56
56 capsules (7 blister cards of 8 capsules)
1 Podhaler device
16.2 Storage and Handling
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)
Protect TOBI Podhaler from moisture.
- o
- TOBI Podhaler capsules should be used with the Podhaler device only. The Podhaler device should not be used with any other capsules.
- o
- Capsules should always be stored in the blister and each capsule should only be removed immediately before use.
- o
- Always use the new Podhaler device provided with each weekly pack.
Keep this and all drugs out of the reach of children.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Important Administration Information
Information on the long-term efficacy and safety of TOBI Podhaler is limited. There is no information in patients with limited pulmonary reserve (FEV1 <25% predicted). Decreased susceptibility of Pseudomonas aeruginosa to tobramycin has been seen with use of TOBI Podhaler. The relationship between in vitro susceptibility test results and clinical outcome with TOBI Podhaler therapy is not clear. Occurrence of decreased susceptibility on treatment should be monitored, and treatment with an alternative therapy should be considered if clinical worsening is observed.
TOBI Podhaler may not be tolerated by all patients. Patients should be instructed to consider alternative therapy if they are unable to tolerate TOBI Podhaler. Patients should be advised to complete a full 28-day course of TOBI Podhaler, even if they are feeling better. After 28 days of therapy, patients should stop TOBI Podhaler therapy for the next 28 days, and then resume therapy for the next 28-day on and 28-day off cycle.
Patients should be advised that if they have been prescribed a 7-day pack of TOBI Podhaler either immediately before or during a 28-day treatment with TOBI Podhaler, then they must count each day of use toward the 28 day on-treatment part of their cycle. Patients should only take a total of 28 consecutive days of treatment during a cycle.
Similarly, patients should be advised that if they have been prescribed a 1-day pack of TOBI Podhaler either immediately before or during a 28-day treatment with TOBI Podhaler, then they must count each day of use toward the 28 day on-treatment part of their cycle. Patients should only take a total of 28 consecutive days of treatment during a cycle.
It is important for patients to understand how to correctly administer TOBI Podhaler capsules using the Podhaler device. It is recommended that caregivers and patients be adequately trained in the proper use of the TOBI Podhaler prior to use. [See Instructions for Use at the end of the Patient Information leaflet.] Caregivers should provide assistance to children using TOBI Podhaler (including preparing the dose for inhalation) particularly for those aged 10 years or younger, and should continue to supervise them until they are able to use the Podhaler device properly without help.
For patients taking several different inhaled medications and/or performing chest physiotherapy, advise the patient regarding the order in which they should take the therapies. It is recommended that TOBI Podhaler be taken last.
Difficulty Breathing:
Advise patients to inform their physicians if they experience shortness of breath or wheezing after administration of Tobi Podhaler. Tobi Podhaler can cause a narrowing of the airway [see Warnings and Precautions (5.1)].
Hearing Loss:
Advise patients to inform their physician if they experience ringing in the ears, dizziness, or any changes in hearing because Tobi Podhaler has been associated with hearing loss [see Warnings and Precautions (5.2)].
Kidney Damage:
Advise patients to inform their physician if they have any history of kidney problems because Tobi Podhaler is in a class of drugs that have caused kidney damage [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity:
Advise pregnant women that aminoglycosides can cause irreversible congenital deafness when administered to a pregnant woman [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1)].
Lactation:
Advise a woman to monitor their breastfed infants for diarrhea and/or bloody stools [see Use in Specific Populations (8.2)].
Cough:
Inform patients that cough was reported with the use of TOBI Podhaler in clinical trials. If coughing that may be experienced with TOBI Podhaler becomes bothersome or cannot be tolerated, advise patients that tobramycin inhalation solution or alternative therapeutic options may be considered.
Patient Information
INSTRUCTIONS FOR USE
TOBI (TOH-bee) Podhaler (POD-hay-ler)
(tobramycin inhalation powder)
- •
- Your healthcare provider should show you or a caregiver how to use TOBI Podhaler the right way before you use it for the first time. Ask your healthcare provider if you have any questions about how to use TOBI Podhaler the right way.
- •
- The recommended dose of TOBI Podhaler is the contents of 4 capsules inhaled by mouth 2 times each day.
- •
- TOBI Podhaler comes in a blister card. Each blister card has 8 TOBI Podhaler capsules: 4 capsules for inhalation in the morning and 4 capsules for inhalation in the evening.
- •
-
You must inhale all of the powdered medicine from all 4 TOBI Podhaler capsules to get the full dose. If all of the powdered TOBI Podhaler medicine is not inhaled, you will not get the full dose.
- o
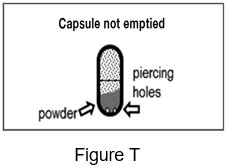
- After you have inhaled 2 times from a capsule, remove the capsule from the capsule chamber and hold the used capsule up to the light and look through it. It should be empty with only a fine coating of powder left on the inside surface of the capsule (See Figure S).
- ▪
- If the capsule is empty, throw it away and continue following the Instructions for Use.
- ▪
- If the capsule is not empty, see the section “What to do with a capsule that has not been emptied” below for instructions.
- •
-
You or a caregiver should tell your healthcare provider as soon as possible if you think you or your child has not received the full TOBI Podhaler dose. Your healthcare provider should show you how to use TOBI Podhaler the right way.
Follow the instructions below for using your TOBI Podhaler. You will breathe in (inhale) the medicine in the TOBI Podhaler capsules using the Podhaler device. If you have any questions, ask your healthcare provider or pharmacist.
TOBI Podhaler is available as a 28-day, 7-day, and 1-day supply package.
Each TOBI Podhaler package contains (See Figure A):- o
-
4 weekly packs (28-day supply), each containing:
- ▪
- 56 capsules (7 blister cards of 8 capsules). Each blister card contains 8 TOBI Podhaler capsules (4 capsules for inhalation in the morning and 4 capsules for inhalation in the evening).
- ▪
- 1 Podhaler device and its storage case
- ▪
- 1 extra reserve Podhaler device (to be used if needed) and its storage case.
-
Or:
- o
-
7-day pack (7-day supply) containing:
- ▪
- 56 capsules (7 blister cards of 8 capsules). Each blister card contains 8 TOBI Podhaler capsules (4 capsules for inhalation in the morning and 4 capsules for inhalation in the evening).
- ▪
- 1 Podhaler device and its storage case.
-
Or:
- o
-
1-day pack (1-day supply) containing:
- ▪
- 8 capsules (1 blister card of 8 capsules). Each blister card contains 8 TOBI Podhaler capsules (4 capsules for inhalation in the morning and 4 capsules for inhalation in the evening).
- ▪
- 1 Podhaler device and its storage case.
Note:
- •
- Do not swallow TOBI Podhaler capsules. The powder in the capsule is for you to inhale using the Podhaler device.
- •
- Only use the Podhaler device that comes in the pack. Do not use TOBI Podhaler capsules with any other device, and do not use the Podhaler device to take any other medicine.
- •
- When you start a new weekly (7-day) pack of capsules, use the new Podhaler device that is supplied in the pack and throw away (discard) the used device and its storage case. Each Podhaler device is only used for 1 week (7 days).
- •
- Always keep the TOBI Podhaler capsules in the blister card. Only remove 1 capsule at a time just before you are going to use it.
- •
- Doses should be inhaled as close to 12 hours apart as possible and not less than 6 hours apart.
- •
- Small pieces of the capsules can get into your mouth and you may be able to feel these pieces on your tongue. These small pieces will not hurt you if you swallow or inhale them.
- •
- The extra (reserve) Podhaler device provided in the 28-day supply package may be used if the Podhaler device:
- o
- is wet, dirty, or broken
- o
- has been dropped
- o
- does not seem to be piercing the capsule properly (see Step 17)
Getting ready:
- •
- Wash and dry your hands (See Figure B).
Preparing your TOBI Podhaler dose
Your Podhaler device comes in a storage case with a lid. The device has a removable mouthpiece, capsule chamber and a button at its base (See Figure C).
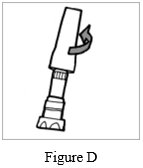
Step 1: Just before use, hold the base of the storage case and unscrew the lid by turning it to the left (counter-clockwise) (See Figure D). Set the lid aside.
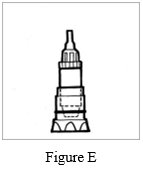
Step 2: Leave the Podhaler device in the base of the storage case while you prepare your dose (See Figure E).
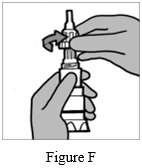
Step 3: Hold the body of the Podhaler device and unscrew the mouthpiece by turning it to the left (counter-clockwise direction) (See Figure F). Set the mouthpiece aside on a clean, dry surface.
Note: Each blister card contains 8 TOBI Podhaler capsules: 4 capsules for inhalation in the morning and 4 capsules for inhalation in the evening.
Step 4:Take 1 blister card and tear the pre-cut lines along the length (See Figure G) then tear at the pre-cut lines along the width (See Figure H).
Step 5: Peel (by rolling back) the foil that covers 1 TOBI Podhaler capsule on the blister card (See Figure I). Always hold the foil close to where you are peeling.
Step 6: Take out 1 TOBI Podhaler capsule from the blister card (See Figure J). Note: Only peel back the foil from 1 capsule at a time and remove the capsule just before you are going to use it in the device because the blister protects the capsule from moisture.
Step 7: Place the TOBI Podhaler capsule in the capsule chamber at the top of the Podhaler device right away (See Figure K). Do not put the capsule directly into the top of the mouthpiece.
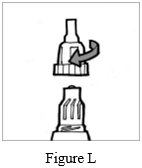
Step 8: Put the mouthpiece back on your Podhaler device and screw the mouthpiece on by turning it to the right (clockwise) until it is tight (See Figure L). Do not overtighten.
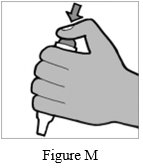
Step 9: Remove the Podhaler device from the base of the case. Hold the Podhaler device with the mouthpiece pointing down. Put your thumb on the blue button and press the blue button all the way down (See Figure M). Let go of the blue button. Do not press the blue button more than 1 time. The chances of the capsule breaking into pieces will be increased if the capsule is accidentally pierced (a hole put in it) more than 1 time.
Taking your TOBI Podhaler dose
Note: You will need to repeat Step 10 to Step 14 for each capsule so you inhale each capsule 2 times to empty the capsule.
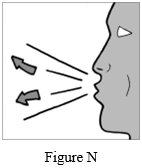
Step 10: Breathe out (exhale) all the way (See Figure N). Do not blow or exhale into the mouthpiece.
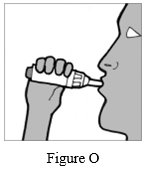
Step 11: Place your mouth over the mouthpiece and close your lips tightly around it (See Figure O).
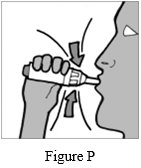
Step 12: Inhale deeply with a single breath (See Figure P).
Step 13: Remove the Podhaler device from your mouth and hold your breath for about 5 seconds.
Step 14: Exhale and take a few normal breaths away from the Podhaler device. Do not blow or exhale into the mouthpiece.
Step 15: Repeat Step 10 through Step 14 using the same capsule.
- •
- You must inhale 2 times from each capsule to empty it.
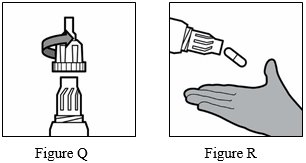
Step 16: Unscrew the mouthpiece by turning it to the left (counterclockwise) and remove the TOBI Podhaler capsule from the capsule chamber (See Figure Q and Figure R below).
Step 17: Hold the used capsule up to the light and look through it. It should be empty with only a fine coating of powder remaining on the inside surface of the capsule (See Figure S). If the capsule is empty, throw it away and go to Step 18.
- If the capsule is not empty, see “What to do with a capsule that has not been emptied” below for instructions.
What to do with a capsule that has not been emptied:
- •
- If the capsule is pierced but still contains more than just a fine coating of powder (See Figure T) you must inhale from it again 2 times:
- o
- Put the capsule back into the Podhaler device capsule chamber with the pierced side of the capsule pointing down.
- o
- Screw the mouthpiece back on the Podhaler device by turning it to the right (clockwise) until it is tight.
- o
- Repeat Step 10 to Step 17.
- •
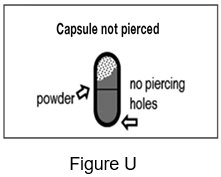
- If the capsule is not pierced (See Figure U) you must pierce it again and inhale from it 2 times:
- o
- Put the capsule back into the Podhaler device capsule chamber.
- o
- Screw the mouthpiece back on the Podhaler device by turning it to the right (clockwise) until it is tight.
- o
- Repeat Step 9 to Step 17 making sure to press the blue button all the way down.
-
Note: If you have tried to pierce the capsule 2 times and it is still not pierced, use the extra (reserve) Podhaler device provided in the TOBI Podhaler package instead (only available in the 28-day supply package). If you need a new device, ask your healthcare provider.
- o
- Prepare the extra (reserve) Podhaler device by following Step 1 to Step 3.
- o
- Then using the same capsule, repeat Step 7 to Step 17.
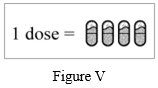
Step 18: Repeat Step 5 to Step 17 for 3 more times until your full dose (4 capsules) has been taken (See Figure V).
After your TOBI Podhaler dose:
Step 19: Do not store the TOBI Podhaler capsules in the Podhaler device.
Step 20: Put the mouthpiece back on to your Podhaler device and screw the mouthpiece on by turning it to the right (clockwise) until it is tight (See Figure L). Do not overtighten.
Step 21: Wipe the mouthpiece with a clean, dry cloth (See Figure W).
- •
- Do not wash the Podhaler device with water. Your Podhaler device needs to stay dry at all times to work the right way.
Step 22: Place your Podhaler device back in the storage case base.
Step 23: Place the lid back on the storage case base and screw the cover on by turning it to the right (clockwise) until it is tight (See Figure X).
How should I store TOBI Podhaler?
- •
- Store your Podhaler device and blister-packaged capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep the TOBI Podhaler capsules and Podhaler device in a dry place.
- •
- Store the Podhaler device tightly closed in its storage case when you are not using it.
- •
- Keep TOBI Podhaler capsules, Podhaler device, and all medicines out of the reach of children.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
© 2020 Mylan Inc.
Tobi® and Podhaler® are registered trademarks of BGP Products Operations GmbH, a Mylan company.
Manufactured for:
Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.
Manufactured by:
Mylan Pharmaceuticals Inc.
150 Industrial Road
San Carlos, CA 94070 U.S.A.
Revised: 7/2020
N:TOBRIP:R2/PI:TOBRIP:R2/IFU:TOBRIP:R2
PRINCIPAL DISPLAY PANEL – 28 mg
NDC 49502-346-24 Rx only
TOBI® Podhaler®
(tobramycin inhalation powder)
28 mg per capsule
For Oral Inhalation Only
Do not swallow TOBI® Podhaler® capsules
TOBI® Podhaler® capsules are for use with the Podhaler® device only
This package contains a 4-week supply of capsules:
4 weekly packs x 56 capsules per pack
Each capsule contains 28 mg tobramycin with 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), calcium chloride, and sulfuric acid (for pH adjustment)
Dosage: See prescribing information.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F); protect from moisture.
Capsules Should Always Be Stored in the Blister and Only Removed Immediately Before Use.
Always store the Podhaler® device in its case.
Keep this and all drugs out of the reach of children.
Contents:
4 weekly packs, each containing:
56 capsules (7 blister cards of 8 capsules)
1 Podhaler® device
Patient information leaflet
1 Reserve Podhaler® device
Prescribing Information
Product of Hungary
Manufactured for:
Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.
Manufactured by:
Mylan Pharmaceuticals Inc.
San Carlos, CA 94070 U.S.A.
© 2019 Mylan Inc.
GTIN: 00349502346248
EXP/LOT
| TOBI PODHALER
tobramycin capsule |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Mylan Specialty L.P. (194775557) |