PLEO NIG- aspergillus niger var. niger solution/ drops
Sanum Kehlbeck GmbH & Co. KG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Oral Homeopathic Medicine
50 doses, each 1 mL
(.03 fl oz)
INDICATIONS
For temporary relief of external itching and soreness in the urogenital region.
DIRECTIONS FOR USE
Snap off top portion of sipping container. Insert glass sipping straw.
DOSAGE
1 SIP, twice weekly.
INGREDIENTS
1 mL contains Aspergillus niger 6X, in a base of purified saline solution.
WARNING
If symptoms persist more than a few days, contact a licensed practitioner. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health care professional before using this product.
Keep this and all medications out of the reach of children. In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Protect from light and heat.
Tamper Evident
Do not use this product if the glass vial is broken or if imprinted security strip on carton is torn.
Made in Germany
Distributed by:
SANUM USA Corp.
1465 Slater Road
Ferndale, WA 98248
Manufactured By:
Sanum-Kehlbeck GmbH & Co. KG
Rev. 09/2002
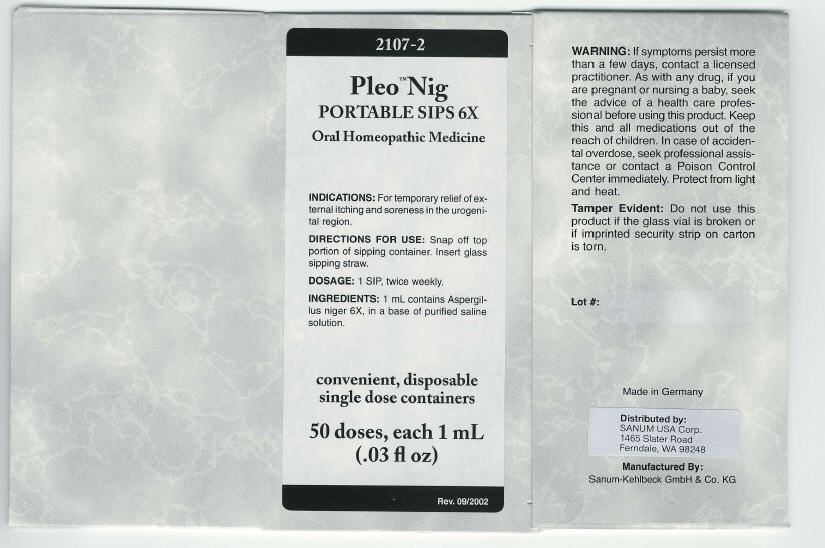
PRINCIPAL DISPLAY PANEL - 1 mL Carton
2107-2
Pleo™ Nig
PORTABLE SIPS 6X
Oral Homeopathic Medicine
INDICATIONS: For temporary relief of ex-
ternal itching and soreness in the urogeni-
tal region.
DIRECTIONS FOR USE: Snap off top
portion of sipping container. Insert glass
sipping straw.
DOSAGE: 1 SIP, twice weekly.
INGREDIENTS: 1 mL contains Aspergil-
lus niger 6X, in a base of purified saline
solution.
convenient, disposable
single dose containers
50 doses, each 1 mL
(.03 fl oz)
Rev. 09/2002
Sanum Kehlbeck GmbH & Co. KG