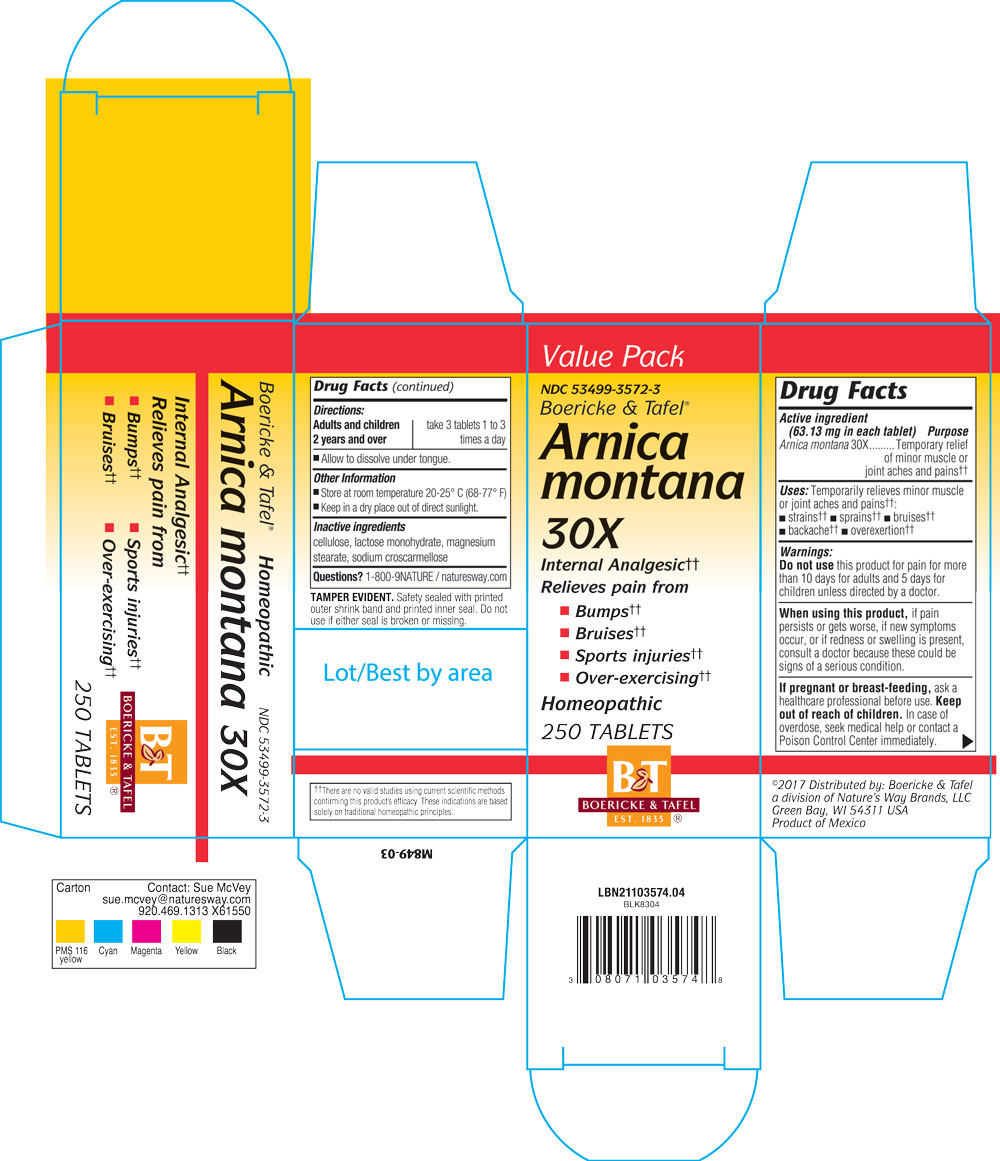
Dosage and Administration
Dosage:
Adults or children over 2 years: Take 3 tablets 1 to 3 times a day.
Allow to dissolve under tongue.
Purpose
Temporarily relieves minor muscle or joint aches and pain from strains, sprains, bruises, backache, overexertion.
Indications & Usage
Uses temporarily relieves minor muscle or joint aches and pains, strains, sprians, bruises, backache, overexertion
Warning
Do not use this product for pain for more than 10 days (for adults) and 5 days (for children) unless directed by a doctor.
When using
When using this product, if pain persists or gets worse, if new symptoms occur, or if redness or swelling is present, consult a doctor because these could be signs of a serious condition.