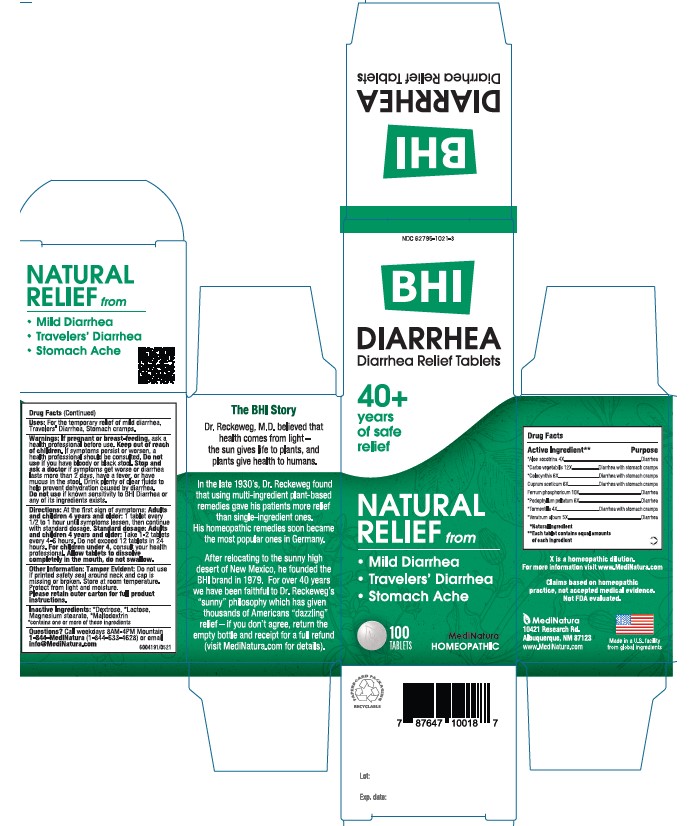
ACTIVE INGREDIENTS
Each tablet contains: *Aloe socotrina 4X, *Carbo vegetabilis 12X *Colocynthis 6X, Cuprum aceticum 6X, Ferrum phosphoricum 10X, *Podophyllum peltatum 6X, *Tormentilla 4X, *Veratrum album 5X 37.5 mg each.
*Natural Ingredients
INACTIVE INGREDIENTS
Inactive Ingredients: **Dextrose, ** Lactose, Magnesium Stearate, **Maltodextrin
**contains one or more of these ingredients.
DIRECTIONS
At first sign of symptoms: Adults and children 4 years and older:
1 tablet every 1/2 to 1 hour until symptoms lessen, then continue with standard dosage.
Standard dosage: Adults and children 4 years and older:
Take 1-2 tablets every 4 to 6 hours. Do not exceed 12 tablets in 24 hours
For children under 4, consult your health professional.
Allow tablets to dissolve completely in the mouth, do not swallow
.
WARNINGS
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. If symptoms persist or worsen, a health professional should be consulted. Do not use if you have bloody or black stool. Stop use and ask a doctor if symptoms get worse or diarrhea lasts more than 2 days, have a fever, or have mucus in the stool. Drink plenty of clear fluids to help prevent dehydration caused by diarrhea. Do not use if known sensitivity to BHI Diarrhea or any of its ingredients exists.