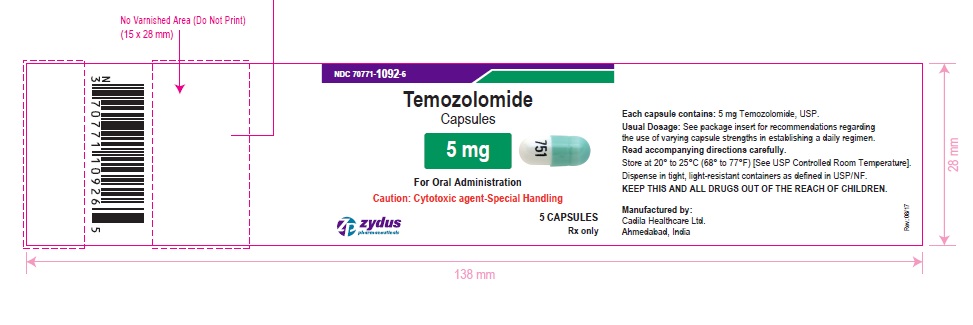
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1092-6 in bottle of 5 capsules
Temozolomide Capsules, 5 mg
Rx only
5 capsules
NDC 70771-1093-6 in bottle of 5 capsules
Temozolomide Capsules, 20 mg
Rx only
5 capsules

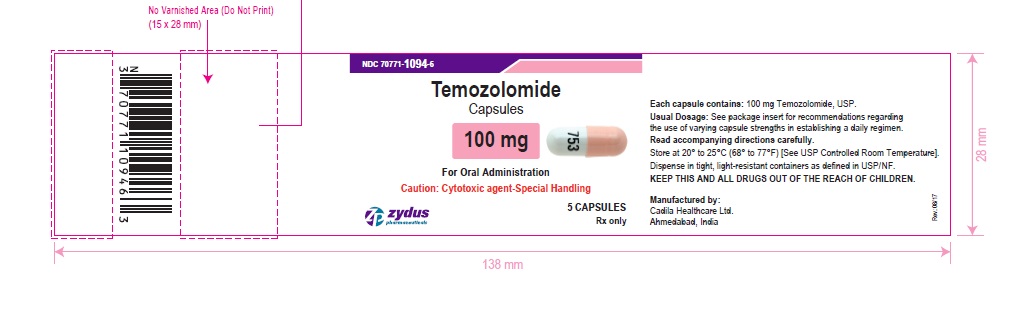
NDC 70771-1094-6 in bottle of 5 capsules
Temozolomide Capsules, 100 mg
Rx only
5 capsules
NDC 70771-1095-6 in bottle of 5 capsules
Temozolomide Capsules, 140 mg
Rx only
5 capsules

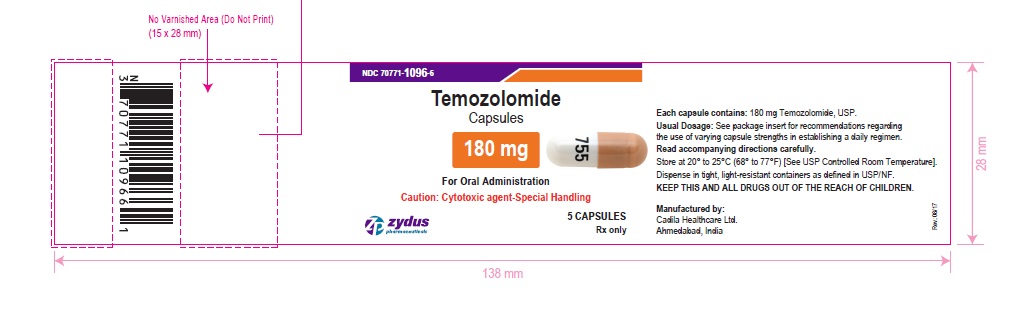
NDC 70771-1096-6 in bottle of 5 capsules
Temozolomide Capsules, 180 mg
Rx only
5 capsules
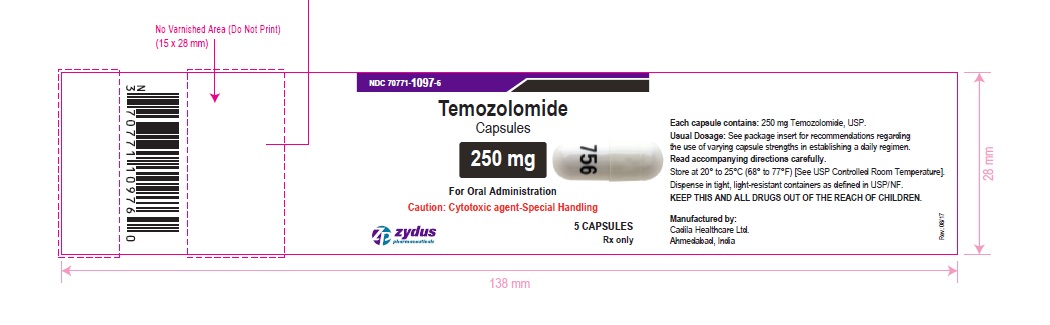
NDC 70771-1097-6 in bottle of 5 capsules
Temozolomide Capsules, 250 mg
Rx only
5 capsules