DESCRIPTION
DDAVP® Tablets (desmopressin acetate) are a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It is chemically defined as follows:
Mol. Wt. 1183.34
Empirical Formula: C46H64N14O12S2∙C2H4O2∙3H2O
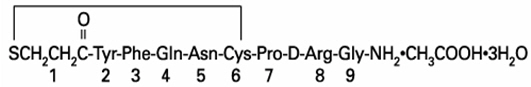
1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate.
DDAVP Tablets contain either 0.1 or 0.2 mg desmopressin acetate. Inactive ingredients include: lactose, potato starch, magnesium stearate and povidone.
CLINICAL PHARMACOLOGY
DDAVP Tablets contain as active substance, desmopressin acetate, a synthetic analogue of the natural hormone arginine vasopressin.
Central Diabetes Insipidus
Dose response studies in patients with diabetes insipidus have demonstrated that oral doses of 0.025 mg to 0.4 mg produced clinically significant antidiuretic effects. In most patients, doses of 0.1 mg to 0.2 mg produced optimal antidiuretic effects lasting up to eight hours. With doses of 0.4 mg, antidiuretic effects were observed for up to 12 hours; measurements beyond 12 hours were not recorded. Increasing oral doses produced dose dependent increases in the plasma levels of DDAVP (desmopressin acetate).
The plasma half-life of DDAVP followed a monoexponential time course with t1/2 values of 1.5 to 2.5 hours which was independent of dose.
The bioavailability of DDAVP oral tablets is about 5% compared to intranasal DDAVP, and about 0.16% compared to intravenous DDAVP. The time to reach maximum plasma DDAVP levels ranged from 0.9 to 1.5 hours following oral or intranasal administration, respectively. Following administration of DDAVP Tablets, the onset of antidiuretic effect occurs at around 1 hour, and it reaches a maximum at about 4 to 7 hours based on the measurement of increased urine osmolality.
The use of DDAVP Tablets in patients with an established diagnosis will result in a reduction in urinary output with an accompanying increase in urine osmolality. These effects usually will allow resumption of a more normal life style, with a decrease in urinary frequency and nocturia.
There are reports of an occasional change in response to the intranasal formulations of DDAVP (DDAVP Nasal Spray and DDAVP Rhinal Tube). Usually, the change occurred over a period of time greater than six months. This change may be due to decreased responsiveness, or to shortened duration of effect. There is no evidence that this effect is due to the development of binding antibodies, but may be due to a local inactivation of the peptide. No lessening of effect was observed in the 46 patients who were treated with DDAVP Tablets for 12 to 44 months and no serum antibodies to desmopressin were detected.
The change in structure of arginine vasopressin to desmopressin acetate resulted in less vasopressor activity and decreased action on visceral smooth muscle relative to enhanced antidiuretic activity. Consequently, clinically effective antidiuretic doses are usually below the threshold for effects on vascular or visceral smooth muscle. In the four long-term studies of DDAVP Tablets, no increases in blood pressure in 46 patients receiving DDAVP Tablets for periods of 12 to 44 months were reported.
In one study, the pharmacodynamic characteristics of DDAVP Tablets and intranasal formulation were compared during an 8-hour dosing interval at steady state. The doses administered to 36 hydrated (water loaded) healthy male adult volunteers every 8 hours were 0.1, 0.2, 0.4 mg orally and 0.01 mg intranasally by rhinal tube. The results are shown in the following table:
| Treatment | Total Urine Volume in mL | Maximum Urine Osmolality in mOsm/kg |
|---|---|---|
| (SE) = Standard error of the mean | ||
| 0.1 mg PO q8h | -3689.3 (149.6) | 514.8 (21.9) |
| 0.2 mg PO q8h | -4429.9 (149.6) | 686.3 (21.9) |
| 0.4 mg PO q8h | -4998.8 (149.6) | 769.3 (21.9) |
| 0.01 mg IN q8h | -4844.9 (149.6) | 754.1 (21.9) |
With respect to the mean values of total urine volume decrease and maximum urine osmolality increase from baseline, the 90% confidence limits estimated that the 0.4 mg and 0.2 mg oral dose produced between 95% and 110% and 84% to 99% of pharmacodynamic activity, respectively, when compared to the 0.01 mg intranasal dose.
While both the 0.2 mg and 0.4 mg oral doses are considered pharmacodynamically similar to the 0.01 mg intranasal dose, the pharmacodynamic data on an inter-subject basis was highly variable and, therefore, individual dosing is recommended.
In another study in diabetes insipidus patients, the pharmacodynamic characteristics of DDAVP Tablets and intranasal formulations were compared over a 12-hour period. Ten fluid-controlled patients under age 18 were administered tablet doses of 0.2 mg and 0.4 mg, and intranasal doses of 0.01 mg and 0.02 mg.
| Treatment | Urine Volume in mL/min | Maximum Urine Osmolality in mOsm/kg |
|---|---|---|
| (SD) = Standard Deviation | ||
| 0.01 mg IN | 0.3 (0.15) | 717.0 (224.63) |
| 0.02 mg IN | 0.3 (0.25) | 761.8 (298.82) |
| 0.2 mg PO | 0.3 (0.12) | 678.3 (147.91) |
| 0.4 mg PO | 0.2 (0.15) | 787.2 (73.34) |
All four dose formulations (0.01 mg IN, 0.02 mg IN, 0.2 mg PO and 0.4 mg PO) have a similar, pronounced pharmacodynamic effect on urine volume and urine osmolality. At two hours after study drug administration, mean urine volume was 4 mL/min and urine osmolality was >500 mOsm/kg. Mean plasma osmolality remained relatively constant over the time course recorded (0 to 12 hours). A statistical separation from baseline did not occur at any dose or time point. In these patients, the 0.2 mg tablets and the 0.01 mg intranasal spray exhibited similar pharmacodynamic profiles as did the 0.4 mg tablets and the 0.02 mg intranasal spray formulation. In another study of adult diabetes insipidus patients previously controlled on DDAVP intranasal spray, after one week of self-titration from spray to tablets, patients' diuresis was controlled with 0.1 mg DDAVP Tablets three times a day.
Primary Nocturnal Enuresis
Two double-blind, randomized, placebo-controlled studies were conducted in 340 patients with primary nocturnal enuresis. Patients were 5-17 years old, and 72% were males. A total of 329 patients were evaluated for efficacy. Patients were evaluated over a two-week baseline period in which the average number of wet nights was 10 (range 4-14). Patients were then randomized to receive 0.2, 0.4, or 0.6 mg of DDAVP or placebo. The pooled results after two weeks are shown in the following table:
| Placebo (n = 85) | 0.2 mg/day (n = 79) | 0.4 mg/day (n = 82) | 0.6 mg/day (n = 83) |
|
|---|---|---|---|---|
| Baseline | 10 (0.3) | 11 (0.3) | 10 (0.3) | 10 (0.3) |
| Reduction from Baseline | 1 (0.3) | 3 (0.4) | 3 (0.4) | 4 (0.4) |
| Percent Reduction from Baseline | 10% | 27% | 30% | 40% |
| p-value vs placebo | —— | <0.05 | <0.05 | <0.05 |
Patients treated with DDAVP Tablets showed a statistically significant reduction in the number of wet nights compared to placebo-treated patients. A greater response was observed with increasing doses up to 0.6 mg.
In a six month, open-label extension study, patients completing the placebo-controlled studies were started on 0.2 mg/day DDAVP, and the dose was progressively increased until the optimal response was achieved (maximum dose 0.6 mg/day). A total of 230 patients were evaluated for efficacy; the average number of wet nights/2 weeks during the untreated baseline period was 10 (range 4-14), and the average duration (SD) of treatment was 4.2 (1.8) months. Twenty-five (25) patients (11%) achieved a complete or near complete response (≤2 wet nights/2 weeks) and did not require titration to the 0.6 mg/day dose. The majority of patients (198 of 230, 86%) were titrated to the highest dose. When all dose groups were combined, 128 (56%) showed at least a 50% reduction from baseline in the number of wet nights/2 weeks, while 87 (38%) patients achieved a complete or near complete response.
Human Pharmacokinetics
DDAVP is mainly excreted in the urine. A pharmacokinetic study conducted in healthy volunteers and patients with mild, moderate, and severe renal impairment (n=24, 6 subjects in each group) receiving single dose desmopressin acetate (2 mcg) injection demonstrated a difference in DDAVP terminal half-life. Terminal half-life significantly increased from 3 hours in normal healthy patients to 9 hours in patients with severe renal impairment. (See CONTRAINDICATIONS.)
INDICATIONS AND USAGE
Central Diabetes Insipidus
DDAVP Tablets are indicated as antidiuretic replacement therapy in the management of central diabetes insipidus and for the management of the temporary polyuria and polydipsia following head trauma or surgery in the pituitary region. DDAVP is ineffective for the treatment of nephrogenic diabetes insipidus.
Patients were selected for therapy based on the diagnosis by means of the water deprivation test, the hypertonic saline infusion test, and/or response to antidiuretic hormone. Continued response to DDAVP can be monitored by measuring urine volume and osmolality.
CONTRAINDICATIONS
DDAVP Tablets are contraindicated in individuals with known hypersensitivity to desmopressin acetate or to any of the components of DDAVP Tablets.
DDAVP is contraindicated in patients with moderate to severe renal impairment (defined as a creatinine clearance below 50 mL/min).
DDAVP is contraindicated in patients with hyponatremia or a history of hyponatremia.
WARNINGS
1. Very rare cases of hyponatremia have been reported from world-wide postmarketing experience in patients treated with DDAVP (desmopressin acetate). DDAVP is a potent antidiuretic which, when administered, may lead to water intoxication and/or hyponatremia. Unless properly diagnosed and treated hyponatremia can be fatal. Therefore, fluid restriction is recommended and should be discussed with the patient and/or guardian. Careful medical supervision is required.
2. When DDAVP Tablets are administered, in particular in pediatric and geriatric patients, fluid intake should be adjusted downward to decrease the potential occurrence of water intoxication and hyponatremia. (See PRECAUTIONS, Pediatric Use and Geriatric Use.) All patients receiving DDAVP therapy should be observed for the following signs of symptoms associated with hyponatremia: headache, nausea/vomiting, decreased serum sodium, weight gain, restlessness, fatigue, lethargy, disorientation, depressed reflexes, loss of appetite, irritability, muscle weakness, muscle spasms or cramps and abnormal mental status such as hallucinations, decreased consciousness and confusion. Severe symptoms may include one or a combination of the following: seizure, coma and/or respiratory arrest. Particular attention should be paid to the possibility of the rare occurrence of an extreme decrease in plasma osmolality that may result in seizures which could lead to coma.
3. DDAVP should be used with caution in patients with habitual or psychogenic polydipsia who may be more likely to drink excessive amounts of water, putting them at greater risk of hyponatremia.
PRECAUTIONS
General
Intranasal formulations of DDAVP at high doses and DDAVP Injection have infrequently produced a slight elevation of blood pressure which disappears with a reduction of dosage. Although this effect has not been observed when single oral doses up to 0.6 mg have been administered, the drug should be used with caution in patients with coronary artery insufficiency and/or hypertensive cardiovascular disease, because of a possible rise in blood pressure.
DDAVP should be used with caution in patients with conditions associated with fluid and electrolyte imbalance, such as cystic fibrosis, heart failure and renal disorders because these patients are prone to hyponatremia.
Rare severe allergic reactions have been reported with DDAVP. Anaphylaxis has been reported rarely with intravenous and intranasal administration of DDAVP but not with DDAVP Tablets.
Drug Interactions
Although the pressor activity of DDAVP is very low compared to its antidiuretic activity, large doses of DDAVP Tablets should be used with other pressor agents only with careful patient monitoring. The concomitant administration of drugs that may increase the risk of water intoxication with hyponatremia, (e.g. tricyclic antidepressants, selective serotonin re-uptake inhibitors, chlorpromazine, opiate analgesics, NSAIDs, lamotrigine and carbamazepine) should be performed with caution.
Carcinogenicity, Mutagenicity, Impairment of Fertility
Studies with DDAVP have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy
Category B
Fertility studies have not been done. Teratology studies in rats and rabbits at doses from 0.05 to 10 mcg/kg/day (approximately 0.1 times the maximum systemic human exposure in rats and up to 38 times the maximum systemic human exposure in rabbits based on surface area, mg/m2) revealed no harm to the fetus due to DDAVP (desmopressin acetate). There are, however, no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Several publications where desmopressin acetate was used in the management of diabetes insipidus during pregnancy are available; these include a few anecdotal reports of congenital anomalies and low birth weight babies. However, no causal connection between these events and desmopressin acetate has been established. A fifteen year Swedish epidemiologic study of the use of desmopressin acetate in pregnant women with diabetes insipidus found the rate of birth defects to be no greater than that in the general population; however, the statistical power of this study is low. As opposed to preparations containing natural hormones, desmopressin acetate in antidiuretic doses has no uterotonic action and the physician will have to weigh the possible therapeutic advantages against the possible risks in each case.
Nursing Mothers
There have been no controlled studies in nursing mothers. A single study in postpartum women demonstrated a marked change in plasma, but little if any change in assayable DDAVP in breast milk following an intranasal dose of 0.01 mg.
It is not known whether the drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when DDAVP is administered to nursing mothers.
Pediatric Use
Central Diabetes Insipidus
DDAVP Tablets (desmopressin acetate) have been used safely in pediatric patients, age 4 years and older, with diabetes insipidus for periods up to 44 months. In younger pediatric patients the dose must be individually adjusted in order to prevent an excessive decrease in plasma osmolality leading to hyponatremia and possible convulsions; dosing should start at 0.05 mg (½ of the 0.1 mg tablet). Use of DDAVP Tablets in pediatric patients requires careful fluid intake restrictions to prevent possible hyponatremia and water intoxication. Fluid restriction should be discussed with the patient and/or guardian. (See WARNINGS.)
Primary Nocturnal Enuresis
DDAVP Tablets have been safely used in pediatric patients age 6 years and older with primary nocturnal enuresis for up to 6 months. Some patients respond to a dose of 0.2 mg; however, increasing responses are seen at doses of 0.4 mg and 0.6 mg. No increase in the frequency or severity of adverse reactions or decrease in efficacy was seen with an increased dose or duration. The dose should be individually adjusted to achieve the best results. Treatment with desmopressin for primary nocturnal enuresis should be interrupted during acute intercurrent illness characterized by fluid and/or electrolyte imbalance (e.g., systemic infections, fever, recurrent vomiting or diarrhea) or under conditions of extremely hot weather, vigorous exercise or other conditions associated with increased water intake.
Geriatric Use
Clinical studies of DDAVP Tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. DDAVP is contraindicated in patients with moderate to severe renal impairment (defined as a creatinine clearance below 50 mL/min). (See CLINICAL PHARMACOLOGY, Human Pharmacokinetics and CONTRAINDICATIONS.)
Use of DDAVP Tablets in geriatric patients requires careful fluid intake restrictions to prevent possible hyponatremia and water intoxication. Fluid restriction should be discussed with the patient. (See WARNINGS.)
ADVERSE REACTIONS
Infrequently, large doses of the intranasal formulations of DDAVP and DDAVP Injection have produced transient headache, nausea, flushing and mild abdominal cramps. These symptoms have disappeared with reduction in dosage.
Central Diabetes Insipidus
In long-term clinical studies in which patients with diabetes insipidus were followed for periods up to 44 months of DDAVP Tablet therapy, transient increases in AST (SGOT) no higher than 1.5 times the upper limit of normal were occasionally observed. Elevated AST (SGOT) returned to the normal range despite continued use of DDAVP Tablets.
Primary Nocturnal Enuresis
The only adverse event occurring in ≥3% of patients in controlled clinical trials with DDAVP Tablets that was probably, possibly, or remotely related to study drug was headache (4% DDAVP, 3% placebo).
Other
The following adverse events have been reported; however their relationship to DDAVP has not been established: abnormal thinking, diarrhea, and edema-weight gain.
See WARNINGS for the possibility of water intoxication and hyponatremia.
Post Marketing: There have been rare reports of hyponatremic convulsions associated with concomitant use with the following medications: oxybutinin and imipramine.
OVERDOSAGE
Signs of overdose may include confusion, drowsiness, continuing headache, problems with passing urine and rapid weight gain due to fluid retention. (See WARNINGS.) In case of overdose, the dose should be reduced, frequency of administration decreased, or the drug withdrawn according to the severity of the condition. There is no known specific antidote for DDAVP. The patient should be observed and treated with appropriate symptomatic therapy.
An oral LD50 has not been established. Oral doses up to 0.2 mg/kg/day have been administered to dogs and rats for 6 months without any significant drug-related toxicities reported. An intravenous dose of 2 mg/kg in mice demonstrated no effect.
DOSAGE AND ADMINISTRATION
Central Diabetes Insipidus
The dosage of DDAVP Tablets must be determined for each individual patient and adjusted according to the diurnal pattern of response. Response should be estimated by two parameters: adequate duration of sleep and adequate, not excessive, water turnover. Patients previously on intranasal DDAVP therapy should begin tablet therapy twelve hours after the last intranasal dose. During the initial dose titration period, patients should be observed closely and appropriate safety parameters measured to assure adequate response. Patients should be monitored at regular intervals during the course of DDAVP Tablet therapy to assure adequate antidiuretic response. Modifications in dosage regimen should be implemented as necessary to assure adequate water turnover. Fluid restriction should be observed. (See WARNINGS, PRECAUTIONS, Pediatric Use and Geriatric Use.)
Adults and Children
It is recommended that patients be started on doses of 0.05 mg (½ of the 0.1 mg tablet) two times a day and individually adjusted to their optimum therapeutic dose. Most patients in clinical trials found that the optimal dosage range is 0.1 mg to 0.8 mg daily, administered in divided doses. Each dose should be separately adjusted for an adequate diurnal rhythm of water turnover. Total daily dosage should be increased or decreased in the range of 0.1 mg to 1.2 mg divided into two or three daily doses as needed to obtain adequate antidiuresis. See Pediatric Use subsection for special considerations when administering desmopressin acetate to pediatric diabetes insipidus patients.
Geriatric Use
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. (See CLINICAL PHARMACOLOGY, Human Pharmacokinetics, CONTRAINDICATIONS, and PRECAUTIONS, Geriatric Use.)
Primary Nocturnal Enuresis
The dosage of DDAVP Tablets must be determined for each individual patient and adjusted according to response. Patients previously on intranasal DDAVP therapy can begin tablet therapy the night following (24 hours after) the last intranasal dose. The recommended initial dose for patients age 6 years and older is 0.2 mg at bedtime. The dose may be titrated up to 0.6 mg to achieve the desired response. Fluid restriction should be observed, and fluid intake should be limited to a minimum from 1 hour before desmopressin acetate administration, until the next morning, or at least 8 hours after administration. (See WARNINGS, PRECAUTIONS, Pediatric Use and Geriatric Use.)
HOW SUPPLIED
| Strength | Size | NDC Code | Color | Markings |
|---|---|---|---|---|
| 0.1 mg | Bottle of 100 | 55566-2600-0 | White |  |
| 0.2 mg | Bottle of 100 | 55566-2700-0 | White | 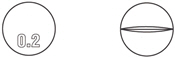 |
Manufactured for:
Ferring Pharmaceuticals Inc.
Parsippany, NJ 07054 USA
Origin Sweden
Rev. 07/2020
XXXXXXXXXX