Synovex® S
(progesterone and estradiol benzoate implants)
200 mg progesterone and 20 mg estradiol benzoate per implant
SUBCUTANEOUS IMPLANTS FOR GROWING BEEF STEERS FED IN CONFINEMENT FOR SLAUGHTER
This product was manufactured by a non-sterilizing process.
TAKE TIME OBSERVE LABEL DIRECTIONS
Important: Read ALL sides of carton
For subcutaneous ear implantation only.
Approved by FDA under NADA # 009-576
Restricted Drug (California) — Use Only as Directed
WITHDRAWAL PERIODS AND RESIDUE WARNINGS
No withdrawal period is required when used according to labeling. Do not use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in dairy cows or in animals intended for subsequent breeding. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. Implant pellets subcutaneously in ear only. Any other location is a violation of Federal Law. Do not attempt salvage of implanted site for human or animal food.
ANIMAL SAFETY WARNINGS
Bulling has occasionally been reported in implanted steers and heifers. Vaginal and rectal prolapse, udder development, ventral edema and elevated tailheads have occasionally been reported in heifers administered SYNOVEX® S implants.
DIRECTIONS
Implant complete contents of one cartridge cell per steer. Approved implantation technique is fully described in the package insert.
NOTE: Never sacrifice careful, clean technique for speed of implantation.
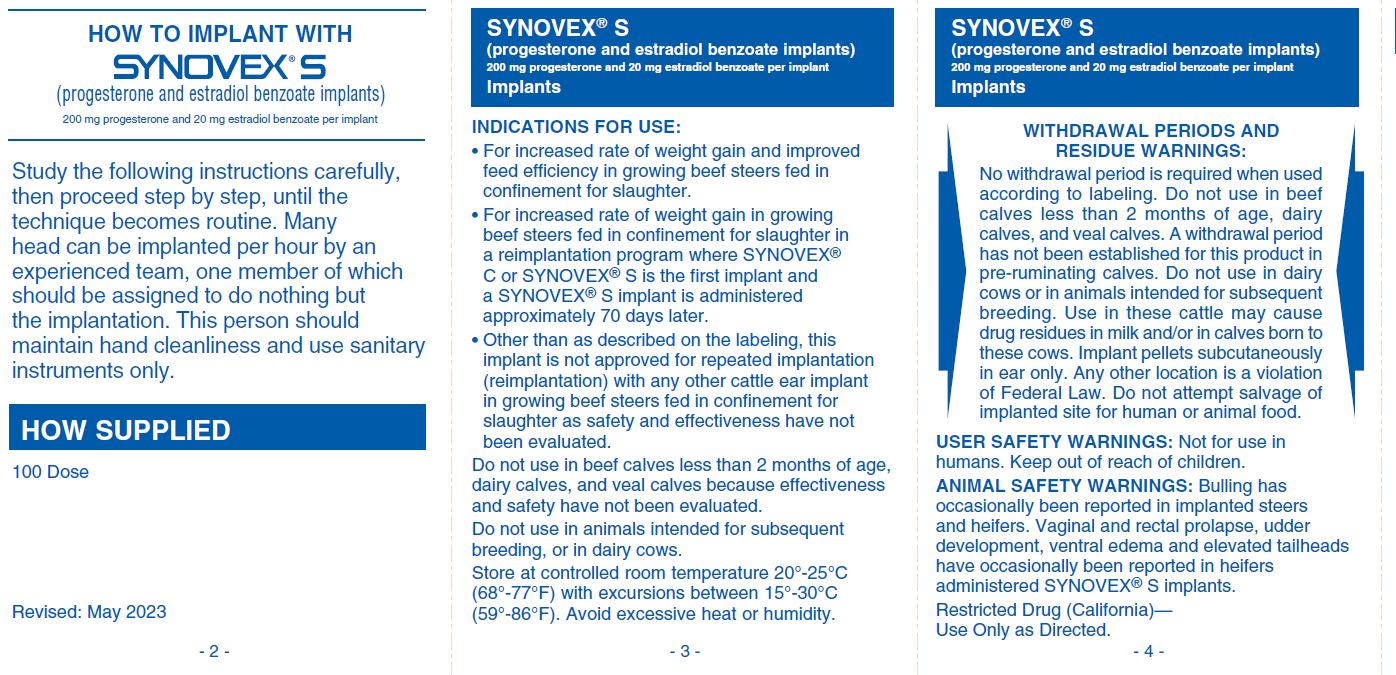
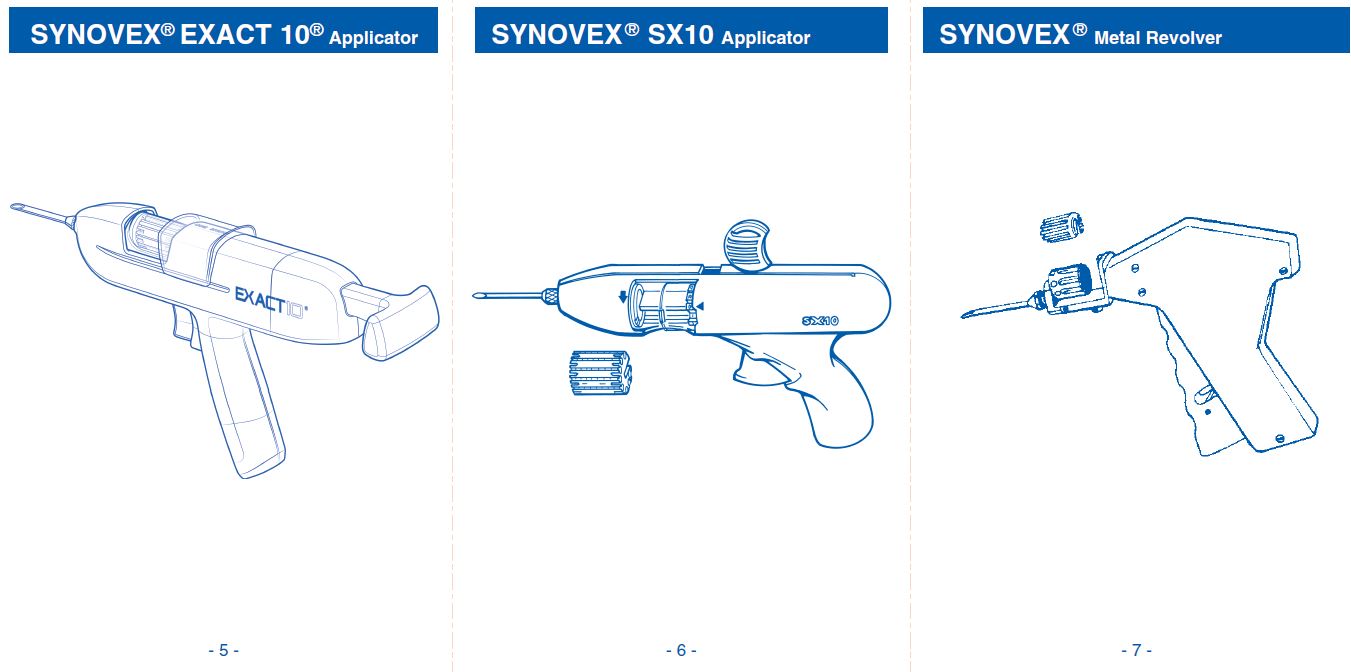
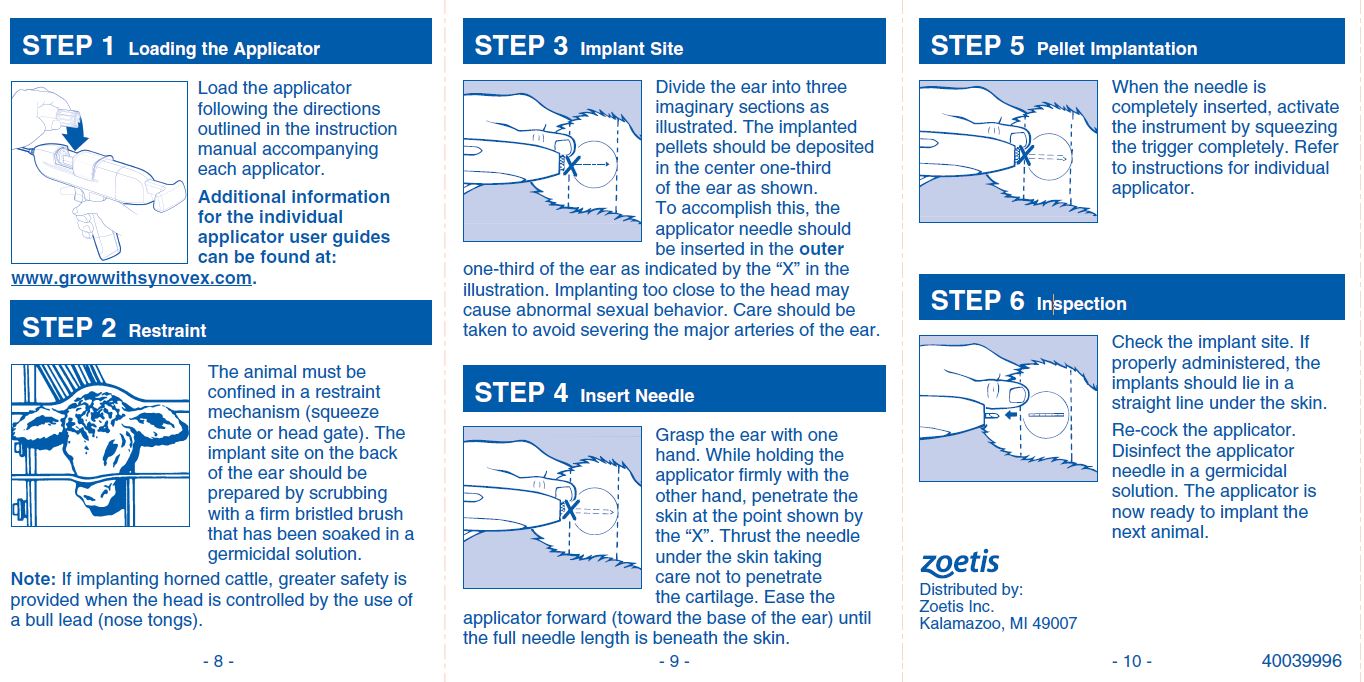
INDICATIONS FOR USE
• For increased rate of weight gain and improved feed efficiency in growing beef steers fed in confinement for slaughter.
• For increased rate of weight gain in growing beef steers fed in confinement for slaughter in a reimplantation program where SYNOVEX® C or SYNOVEX® S is the first implant and a SYNOVEX® S implant is administered approximately 70 days later.
• Other than as described on the labeling, this implant is not approved for repeated implantation (reimplantation) with any other cattle ear implant in growing beef steers fed in confinement for slaughter as safety and effectiveness have not been evaluated.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves because effectiveness and safety have not been evaluated.
Do not use in animals intended for subsequent breeding, or in dairy cows.
Storage
Store at controlled room temperature 20°-25°C (68°-77°F) with excursions between 15°-30°C (59°-86°F). Avoid excessive heat or humidity.
QUESTIONS/COMMENTS?
For a copy of the Safety Data Sheet or to report side effects, contact Zoetis Inc. at 1-888-963-8471. For additional information about reporting side effects for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
