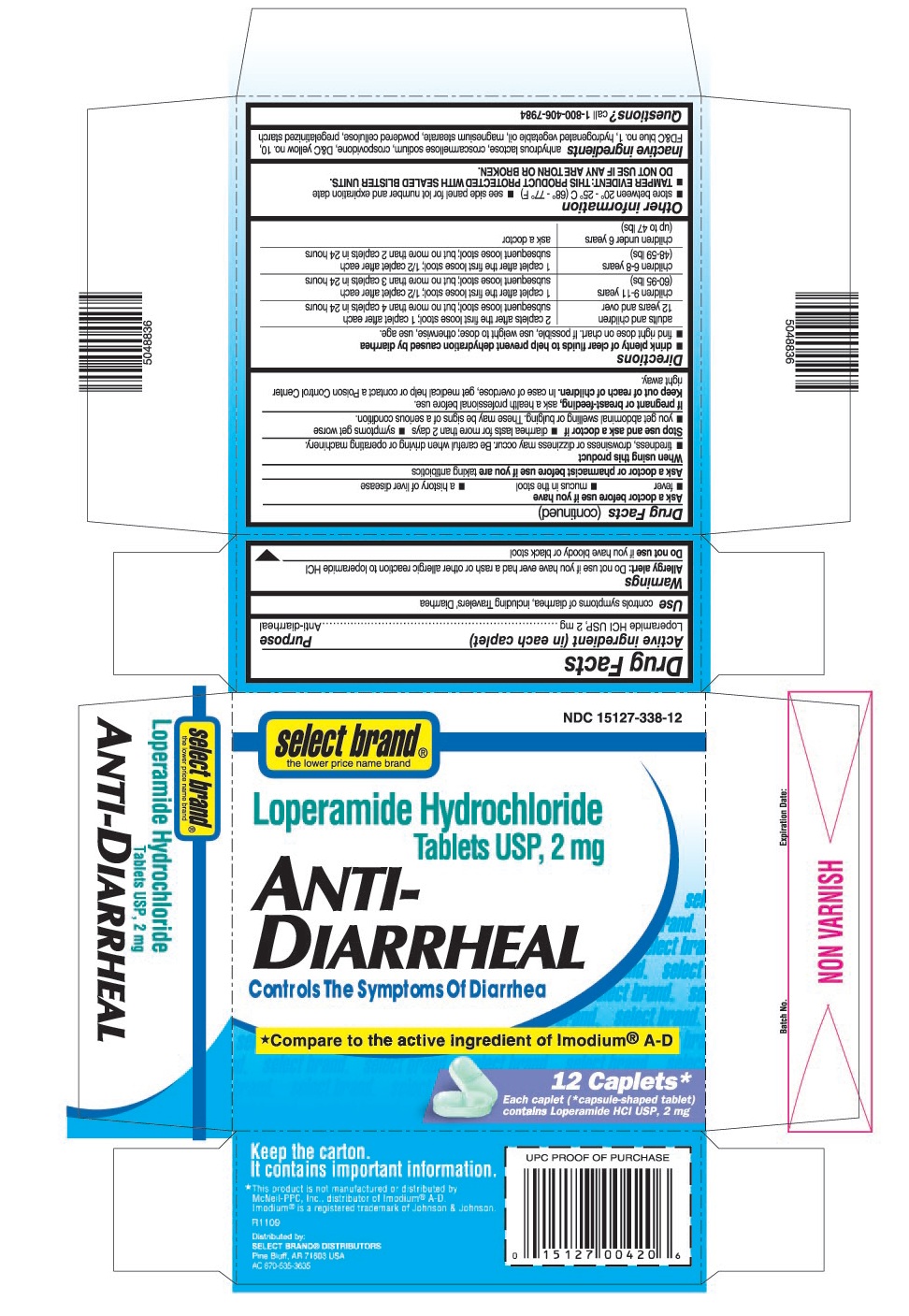
ACTIVE INGREDIENT (IN EACH CAPLET)
Loperamide HCI USP, 2 mg
USES
Controls symptoms of diarrhea, including Travelers’ Diarrhea
WARNINGS
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCI
Do not use
If you have bloody or black stool
Ask a doctor before use if you have
- fever
- mucus in the stool
- a history of liver disease
Ask a doctor or pharmacist before use if you are
Taking antibiotics
When using this product
- tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
Stop use and ask a doctor if
- diarrhea lasts for more than 2 days
- symptoms get worse
- you get abdominal swelling or bulging. These may be signs of a serious condition.
If pregnant or breast-feeding
Ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
-
drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over | 2 caplets after the first loose stool;
1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
children 9-11 years
(60-95 lbs) | 1 caplet after the first loose stool;
½ caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
children 6-8 years
(48-59 lbs) | 1 caplet after the first loose stool;
½ caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
children under 6 years
(up to 47 lbs) | ask a doctor |
OTHER INFORMATION
- store between 20° – 25° C (68° – 77° F)
- see side panel for lot number and expiration date
-
TAMPER EVIDENT: THIS PRODUCT PROTECTED WITH SEALED BLISTER UNITS. DO NOT USE IF ANY ARE TORN OR BROKEN.
INACTIVE INGREDIENTS
Anhydrous lactose, croscarmellose sodium, crospovidone, D&C yellow no.10, FD&C blue no.1, hydrogenated vegetable oil, magnesium stearate, powdered cellulose, pregelatinized starch
QUESTIONS?
Call 1800-406-7984
PRINCIPAL DISPLAY PANEL
select brand®
NDC 15127-338-12
Loperamide Hydrochloride Tablets USP, 2 mg
ANTI-DIARRHEAL
Controls The Symptoms Of Diarrhea
٭Compare to the active ingredient of Imodium®A-D
12 Caplets*
Each Caplet (*capsule-shaped tablet) contains Loperamide HCl USP, 2 mg
5048836/R1109