Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- do not wear a contact lens if your eye is red
Stop use and ask a doctor if you experience:
- eye pain
- changes in vision
- increased redness of the eye
- itching worsens or lasts for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
adults and children 2 years of age and older:
- put 1 drop in the affected eye(s) once daily
-
do not use more than 1 drop in each eye per day
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- replace cap after each use
- children under 2 years of age: consult a doctor
Inactive ingredients
benzalkonium chloride 0.015%, boric acid, hydrochloric acid/sodium hydroxide (to adjust pH), hydroxypropylgamma- cyclodextrin, hypromellose, mannitol, polyethylene glycol 400, povidone, and purified water
PRINCIPAL DISPLAY PANEL
EXTRA STRENGTH
Pataday®
ONCE DAILY RELIEF
Olopatadine hydrochloride
ophthalmic solution 0.7%
Antihistamine
Eye Allergy Itch Relief
FULL 24 HOUR
Works in Minutes
Relief from Allergens:
• Pet Dander
• Pollen
• Grass
• Ragweed
Alcon
STERILE
2.5 mL (0.085 FL OZ)
EXTRA STRENGTH
Pataday®
ONCE DAILY RELIEF
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with Alcon around the neck. Do not use if seal is damaged or missing at time of purchase.
30 DAY SUPPLY
Eye Allergy Itch Relief
Works in Minutes
For Ages 2 and Older
________Fill Line________
Alcon Laboratories, Inc.
6201 South Freeway
Fort Worth, Texas 76134
Country of Origin: Japan
ACTUAL SIZE
NDC: 0065-0816-04
300038456-0620
LOT: EXP.:
3187

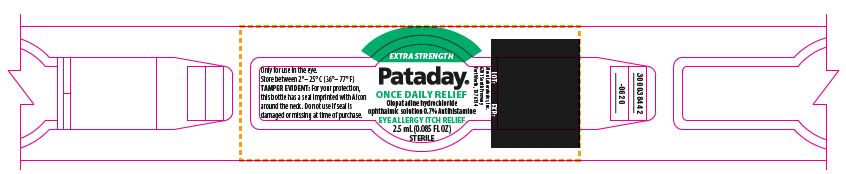
EXTRA STRENGTH
Pataday®
ONCE DAILY RELIEF
Olopatadine hydrochloride
ophthalmic solution 0.7% Antihistamine
2.5 mL (0.085 FL OZ)
STERILE
Only for use in the eye.
Store between 2°– 25° C (36°– 77° F)
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with Alcon around the neck. Do not use if seal is damaged or missing at time of purchase.
Alcon Laboratories, Inc.
6201 South Freeway
Fort Worth, TX 76134
LOT: EXP.:
300038442-0620