FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
LANTUS is indicated to improve glycemic control in adults and pediatric patients with type 1 diabetes mellitus and in adults with type 2 diabetes mellitus.
Important Limitations of Use:
- LANTUS is not recommended for the treatment of diabetic ketoacidosis. Intravenous short-acting insulin is the preferred treatment for this condition.
2. DOSAGE AND ADMINISTRATION
2.1 Dosing
LANTUS is a recombinant human insulin analog for once daily subcutaneous administration with potency that is approximately the same as the potency of human insulin. LANTUS exhibits a relatively constant glucose-lowering profile over 24 hours that permits once-daily dosing.
LANTUS may be administered at any time during the day. LANTUS should be administered subcutaneously once a day at the same time every day. The dose of LANTUS must be individualized based on clinical response. Blood glucose monitoring is essential in all patients receiving insulin therapy.
Patients adjusting the amount or timing of dosing with LANTUS, should only do so under medical supervision with appropriate glucose monitoring .] [see Warnings and Precautions (5.1)
In patients with type 1 diabetes, LANTUS must be used in regimens with short-acting insulin.
The intended duration of activity of LANTUS is dependent on injection into subcutaneous tissue . LANTUS should not be administered intravenously or via an insulin pump. Intravenous administration of the usual subcutaneous dose could result in severe hypoglycemia . [see ] Clinical pharmacology (12.2)[see ] Warnings and Precautions (5.3)
As with all insulins, injection sites should be rotated within the same region (abdomen, thigh, or deltoid) from one injection to the next to reduce the risk of lipodystrophy [See ]. Adverse Reactions (6.1)
In clinical studies, there was no clinically relevant difference in insulin glargine absorption after abdominal, deltoid, or thigh subcutaneous administration. As for all insulins, the rate of absorption, and consequently the onset and duration of action, may be affected by exercise and other variables, such as stress, intercurrent illness, or changes in co-administered drugs or meal patterns .
2.2 Initiation of LANTUS therapy
The recommended starting dose of LANTUS in patients with type 1 diabetes should be approximately one-third of the total daily insulin requirements. Short-acting, premeal insulin should be used to satisfy the remainder of the daily insulin requirements.
The recommended starting dose of LANTUS in patients with type 2 diabetes who are not currently treated with insulin is 10 units (or 0.2 Units/kg) once daily, which should subsequently be adjusted to the patient's needs.
The dose of LANTUS should be adjusted according to blood glucose measurements. The dosage of LANTUS should be individualized under the supervision of a healthcare provider in accordance with the needs of the patient.
2.3 Converting to LANTUS from other insulin therapies
If changing from a treatment regimen with an intermediate-or long-acting insulin to a regimen with LANTUS, the amount and timing of shorter-acting insulins and doses of any oral anti-diabetic drugs may need to be adjusted.
- If transferring patients from once-daily NPH insulin to once-daily LANTUS, the recommended initial LANTUS dose is the same as the dose of NPH that is being discontinued.
- If transferring patients from twice-daily NPH insulin to once-daily LANTUS, the recommended initial LANTUS dose is 80% of the total NPH dose that is being discontinued. This dose reduction will lower the likelihood of hypoglycemia [ see ]. Warnings and Precautions (5.3)
3. DOSAGE FORMS AND STRENGTHS
LANTUS solution for injection 100 Units per mL is available as:
- -
- 10 mL Vial (1000 Units/10 mL)
- -
- 3 mL SoloStar disposable insulin device (300 Units/3 mL) ®
4. CONTRAINDICATIONS
LANTUS is contraindicated
- In patients with hypersensitivity to LANTUS or one of its excipients [See ]. Warnings and Precautions (5.4)
5. WARNINGS AND PRECAUTIONS
5.1 Dosage adjustment and monitoring
Glucose monitoring is essential for all patients receiving insulin therapy. Changes to an insulin regimen should be made cautiously and only under medical supervision.
Changes in insulin strength, manufacturer, type, or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral anti-diabetic treatment.
As with all insulin preparations, the time course of action for LANTUS may vary in different individuals or at different times in the same individual and is dependent on many conditions, including the local blood supply, local temperature, and physical activity.
5.2 Administration
Do not administer LANTUS intravenously or via an insulin pump. The intended duration of activity of LANTUS is dependent on injection into subcutaneous tissue
Intravenous administration of the usual subcutaneous dose could result in severe hypoglycemia . [see ] Warnings and Precautions (5.3)
Do not dilute or mix LANTUS with any other insulin or solution. If LANTUS is diluted or mixed, the solution may become cloudy, and the pharmacokinetic or pharmacodynamic profile (e.g., onset of action, time to peak effect) of LANTUS and the mixed insulin may be altered in an unpredictable manner. When LANTUS and regular human insulin were mixed immediately before injection in dogs, a delayed onset of action and a delayed time to maximum effect for regular human insulin was observed. The total bioavailability of the mixture was also slightly decreased compared to separate injections of LANTUS and regular human insulin. The relevance of these observations in dogs to humans is unknown.
Do not share disposable or reusable insulin devices or needles between patients, because doing so carries a risk for transmission of blood-borne pathogens.
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction of insulin, including LANTUS. The risk of hypoglycemia increases with intensive glycemic control. Patients must be educated to recognize and manage hypoglycemia. Severe hypoglycemia can lead to unconsciousness or convulsions and may result in temporary or permanent impairment of brain function or death. Severe hypoglycemia requiring the assistance of another person or parenteral glucose infusion or glucagon administration has been observed in clinical trials with insulin, including trials with LANTUS.
The timing of hypoglycemia usually reflects the time-action profile of the administered insulin formulations. Other factors such as changes in food intake (e.g., amount of food or timing of meals), exercise, and concomitant medications may also alter the risk of hypoglycemia [ ]. See Drug Interactions (7)
The prolonged effect of subcutaneous LANTUS may delay recovery from hypoglycemia. Patients being switched from twice daily NPH insulin to once-daily LANTUS should have their initial LANTUS dose reduced by 20% from the previous total daily NPH dose to reduce the risk of hypoglycemia [see ]. Dosage and Administration (2.3)
As with all insulins, use caution in patients with hypoglycemia unawareness and in patients who may be predisposed to hypoglycemia (e.g., the pediatric population and patients who fast or have erratic food intake). The patient's ability to concentrate and react may be impaired as a result of hypoglycemia. This may present a risk in situations where these abilities are especially important, such as driving or operating other machinery.
Early warning symptoms of hypoglycemia may be different or less pronounced under certain conditions, such as longstanding diabetes, diabetic neuropathy, use of medications such as beta-blockers, or intensified glycemic control. These situations may result in severe hypoglycemia (and, possibly, loss of consciousness) prior to the patient's awareness of hypoglycemia.
5.4 Hypersensitivity and allergic reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including LANTUS.
5.5 Renal impairment
Due to its long duration of action, Lantus is not recommended during periods of rapidly declining renal function because of the risk for prolonged hypoglycemia.
Although studies have not been performed in patients with diabetes and renal impairment, a reduction in the LANTUS dose may be required in patients with renal impairment because of reduced insulin metabolism, similar to observations found with other insulins. [See ]. Clinical Pharmacology (12.3)
5.6 Hepatic impairment
Due to its long duration of action, Lantus is not recommended during periods of rapidly declining hepatic function because of the risk for prolonged hypoglycemia.
Although studies have not been performed in patients with diabetes and hepatic impairment, a reduction in the LANTUS dose may be required in patients with hepatic impairment because of reduced capacity for gluconeogenesis and reduced insulin metabolism, similar to observations found with other insulins. [See ]. Clinical Pharmacology (12.3)
5.7 Drug interactions
Some medications may alter insulin requirements and subsequently increase the risk for hypoglycemia or hyperglycemia [See ]. Drug Interactions (7)
5.8 Fluid retention and heart failure with concomitant use of PPAR-gamma agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including LANTUS, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
6. ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere:
- Hypoglycemia [See ] Warnings and Precautions (5.3)
- Hypersensitivity and allergic reactions [See ] Warnings and Precautions (5.4)
6.1 Clinical trial experience
Because clinical trials are conducted under widely varying designs, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice.
The frequencies of treatment-emergent adverse events during LANTUS clinical trials in patients with type 1 diabetes mellitus and type 2 diabetes mellitus are listed in the tables below.
| LANTUS, % (n=1257)
| NPH, % (n=1070)
|
|
|---|---|---|
|
||
| Upper respiratory tract infection | 22.4 | 23.1 |
| Infection * | 9.4 | 10.3 |
| Accidental injury | 5.7 | 6.4 |
| Headache | 5.5 | 4.7 |
| LANTUS, % (n=849)
| NPH, % (n=714)
|
|
|---|---|---|
|
||
| Upper respiratory tract infection | 11.4 | 13.3 |
| Infection * | 10.4 | 11.6 |
| Retinal vascular disorder | 5.8 | 7.4 |
| LANTUS, % (n=514)
| NPH, % (n=503)
|
|
|---|---|---|
| Upper respiratory tract infection | 29.0 | 33.6 |
| Edema peripheral | 20.0 | 22.7 |
| Hypertension | 19.6 | 18.9 |
| Influenza | 18.7 | 19.5 |
| Sinusitis | 18.5 | 17.9 |
| Cataract | 18.1 | 15.9 |
| Bronchitis | 15.2 | 14.1 |
| Arthralgia | 14.2 | 16.1 |
| Pain in extremity | 13.0 | 13.1 |
| Back pain | 12.8 | 12.3 |
| Cough | 12.1 | 7.4 |
| Urinary tract infection | 10.7 | 10.1 |
| Diarrhea | 10.7 | 10.3 |
| Depression | 10.5 | 9.7 |
| Headache | 10.3 | 9.3 |
| LANTUS, % (n=174)
| NPH, % (n=175)
|
|
|---|---|---|
|
||
| Infection * | 13.8 | 17.7 |
| Upper respiratory tract infection | 13.8 | 16.0 |
| Pharyngitis | 7.5 | 8.6 |
| Rhinitis | 5.2 | 5.1 |
- Severe Hypoglycemia
Hypoglycemia is the most commonly observed adverse reaction in patients using insulin, including LANTUS . Tables 5, and 6 and 7 summarize the incidence of severe hypoglycemia in the LANTUS individual clinical trials. Severe symptomatic hypoglycemia was defined as an event with symptoms consistent with hypoglycemia requiring the assistance of another person and associated with either a blood glucose below 50 mg/dL (≤56 mg/dL in the 5-year trial and ≤36 mg/dL in the ORIGIN trial) or prompt recovery after oral carbohydrate, intravenous glucose or glucagon administration. [See ] Warnings and Precautions (5.3)
The proportion of patients experiencing severe symptomatic hypoglycemia in the LANTUS clinical trials [ ] in adults with type 1 diabetes and type 2 diabetes were comparable for all treatment regimens (see and ). In the pediatric phase 3 clinical trial, children and adolescents with type 1 diabetes had a higher incidence of severe symptomatic hypoglycemia in the two treatment groups compared to the adult trials with type 1 diabetes. See Clinical Studies (14)Tables 56
| Study A Type 1 Diabetes Adults 28 weeks In combination with regular insulin
| Study B Type 1 Diabetes Adults 28 weeks In combination with regular insulin
| Study C Type 1 Diabetes Adults 16 weeks In combination with insulin lispro
| Study D Type 1 Diabetes Pediatrics 26 weeks In combination with regular insulin
|
|||||
|---|---|---|---|---|---|---|---|---|
| LANTUS | NPH | LANTUS | NPH | LANTUS | NPH | LANTUS | NPH | |
| Percent of patients (n/total N) | 10.6 (31/292)
| 15.0 (44/293)
| 8.7 (23/264)
| 10.4 (28/270)
| 6.5 (20/310)
| 5.2 (16/309)
| 23.0 (40/174)
| 28.6 (50/175)
|
| Study E Type 2 Diabetes Adults 52 weeks In combination with oral agents
| Study F Type 2 Diabetes Adults 28 weeks In combination with regular insulin
| Study G Type 2 Diabetes Adults 5 years In combination with regular insulin
|
||||
|---|---|---|---|---|---|---|
| LANTUS | NPH | LANTUS | NPH | LANTUS | NPH | |
| Percent of patients (n/total N)
| 1.7 (5/289)
| 1.1 (3/281)
| 0.4 (1/259)
| 2.3 (6/259)
| 7.8 (40/513)
| 11.9 (60/504)
|
Table 7 displays the proportion of patients experiencing severe symptomatic hypoglycemia in the Lantus and Standard Care groups in the ORIGIN Trial [ ]. see Adverse Reactions (cardiovascular safety)
| Median duration of follow-up: 6.2 years
ORIGIN Trial
|
||
|---|---|---|
| LANTUS | Standard Care | |
| Percent of patients (n/total N)
| 5.6 (352/6231)
| 1.8 (113/6273)
|
- Retinopathy
Retinopathy was evaluated in the LANTUS clinical studies by analysis of reported retinal adverse events and fundus photography. The numbers of retinal adverse events reported for LANTUS and NPH insulin treatment groups were similar for patients with type 1 and type 2 diabetes.
LANTUS was compared to NPH insulin in a 5-year randomized clinical trial that evaluated the progression of retinopathy as assessed with fundus photography using a grading protocol derived from the Early Treatment Diabetic Retinopathy Scale (ETDRS). Patients had type 2 diabetes (mean age 55 yrs) with no (86%) or mild (14%) retinopathy at baseline. Mean baseline HbA1c was 8.4%. The primary outcome was progression by 3 or more steps on the ETDRS scale at study endpoint. Patients with pre-specified post-baseline eye procedures (pan-retinal photocoagulation for proliferative or severe nonproliferative diabetic retinopathy, local photocoagulation for new vessels, and vitrectomy for diabetic retinopathy) were also considered as 3-step progressors regardless of actual change in ETDRS score from baseline. Retinopathy graders were blinded to treatment group assignment. The results for the primary endpoint are shown in Table 8 for both the per-protocol and Intent-to-Treat populations, and indicate similarity of Lantus to NPH in the progression of diabetic retinopathy as assessed by this outcome.
| Lantus (%) | NPH (%) | Difference (SE) *,† | 95% CI for difference | |
|---|---|---|---|---|
| Per-protocol | 53/374 (14.2%) | 57/363 (15.7%) | -2.0% (2.6%) | -7.0% to +3.1% |
| Intent-to-Treat | 63/502 (12.5%) | 71/487 (14.6%) | - 2.1% (2.1%) | -6.3% to +2.1% |
- Insulin initiation and intensification of glucose control
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral neuropathy. However, long-term glycemic control decreases the risk of diabetic retinopathy and neuropathy.
- Lipodystrophy
Long-term use of insulin, including LANTUS, can cause lipodystrophy at the site of repeated insulin injections. Lipodystrophy includes lipohypertrophy (thickening of adipose tissue) and lipoatrophy (thinning of adipose tissue), and may affect insulin absorption. Rotate insulin injection or infusion sites within the same region to reduce the risk of lipodystrophy. [See ]. Dosage and Administration (2.1)
- Weight gain
Weight gain can occur with insulin therapy, including LANTUS, and has been attributed to the anabolic effects of insulin and the decrease in glucosuria.
- Peripheral Edema
Insulin, including LANTUS, may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
- Allergic Reactions
Local Allergy
As with any insulin therapy, patients taking LANTUS may experience injection site reactions, including redness, pain, itching, urticaria, edema, and inflammation. In clinical studies in adult patients, there was a higher incidence of treatment-emergent injection site pain in LANTUS-treated patients (2.7%) compared to NPH insulin-treated patients (0.7%). The reports of pain at the injection site did not result in discontinuation of therapy.
Rotation of the injection site within a given area from one injection to the next may help to reduce or prevent these reactions. In some instances, these reactions may be related to factors other than insulin, such as irritants in a skin cleansing agent or poor injection technique. Most minor reactions to insulin usually resolve in a few days to a few weeks.
- Antibody production
All insulin products can elicit the formation of insulin antibodies. The presence of such insulin antibodies may increase or decrease the efficacy of insulin and may require adjustment of the insulin dose. In phase 3 clinical trials of LANTUS, increases in titers of antibodies to insulin were observed in NPH insulin and insulin glargine treatment groups with similar incidences.
- Cardiovascular Safety
The Outcome Reduction with Initial Glargine Intervention trial (i.e., ORIGIN) was an open-label, randomized, 2-by-2, factorial design study. One intervention in ORIGIN compared the effect of LANTUS to standard care on major adverse cardiovascular outcomes in 12,537 participants ≥ 50 years of age with abnormal glucose levels [i.e., impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT)] or early type 2 diabetes mellitus and established cardiovascular (i.e., CV) disease or CV risk factors at baseline.
The objective of the trial was to demonstrate that LANTUS use could significantly lower the risk of major cardiovascular outcomes compared to standard care. Two co-primary composite cardiovascular endpoints were used in ORIGIN. The first co-primary endpoint was the time to first occurrence of a major adverse cardiovascular event defined as the composite of CV death, nonfatal myocardial infarction and nonfatal stroke. The second co-primary endpoint was the time to the first occurrence of CV death or nonfatal myocardial infarction or nonfatal stroke or revascularization procedure or hospitalization for heart failure.
Participants were randomized to either LANTUS (N=6264) titrated to a goal fasting plasma glucose of ≤ 95 mg/dL or to standard care (N=6273). Anthropometric and disease characteristics were balanced at baseline. The mean age was 64 years and 8% of participants were 75 years of age or older. The majority of participants were male (65%). Fifty nine percent were Caucasian, 25% were Latin, 10% were Asian and 3% were Black. The median baseline BMI was 29 kg/m . Approximately 12% of participants had abnormal glucose levels (IGT and/or IFG) at baseline and 88% had type 2 diabetes. For patients with type 2 diabetes, 59% were treated with a single oral antidiabetic drug, 23% had known diabetes but were on no antidiabetic drug and 6% were newly diagnosed during the screening procedure. The mean HbA1c (SD) at baseline was 6.5% (1.0). Fifty nine percent of participants had had a prior cardiovascular event and 39% had documented coronary artery disease or other cardiovascular risk factors. 2
Vital status was available for 99.9% and 99.8% of participants randomized to LANTUS and standard care respectively at end of trial. The median duration of follow-up was 6.2 years [range: 8 days to 7.9 years]. The mean HbA1c (SD) at the end of the trial was 6.5% (1.1) and 6.8% (1.2) in the LANTUS and standard care group respectively. The median dose of LANTUS at end of trial was 0.45 U/kg. Eighty-one percent of patients randomized to LANTUS were using LANTUS at end of the study. The mean change in body weight from baseline to the last treatment visit was 2.2 kg greater in the LANTUS group than in the standard care group.
Overall, the incidence of major adverse cardiovascular outcomes was similar between groups (see ). All-cause mortality was also similar between groups. Table 9
| LANTUS N=6264
| Standard Care N=6273
| LANTUS vs Standard Care | |
|---|---|---|---|
| n (Events per 100 PY)
| n (Events per 100 PY)
| Hazard Ratio (95% CI) | |
| Co-primary endpoints | |||
| CV death, nonfatal myocardial infarction, or nonfatal stroke | 1041 (2.9)
| 1013 (2.9)
| 1.02 (0.94, 1.11) |
| CV death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure or revascularization procedure | 1792 (5.5)
| 1727 (5.3)
| 1.04 (0.97, 1.11) |
| Components of co-primary endpoints | |||
| CV death | 580 | 576 | 1.00 (0.89, 1.13) |
| Myocardial Infarction (fatal or non-fatal) | 336 | 326 | 1.03 (0.88, 1.19) |
| Stroke (fatal or non-fatal) | 331 | 319 | 1.03 (0.89, 1.21) |
| Revascularizations | 908 | 860 | 1.06 (0.96, 1.16) |
| Hospitalization for heart failure | 310 | 343 | 0.90 (0.77, 1.05) |
- Cancer
In the ORIGIN trial, the overall incidence of cancer (all types combined) or death from cancer (Table 10) was similar between treatment groups.
| LANTUS N=6264
| Standard Care N=6273
| LANTUS vs Standard Care | |
|---|---|---|---|
| n (Events per 100 PY)
| n (Events per 100 PY)
| Hazard Ratio (95% CI) | |
| Cancer endpoints | |||
| Any cancer event (new or recurrent) | 559 (1.56)
| 561 (1.56)
| 0.99 (0.88, 1.11) |
| New cancer events | 524 (1.46)
| 535 (1.49)
| 0.96 (0.85, 1.09) |
| Death due to Cancer | 189 (0.51)
| 201 (0.54)
| 0.94 (0.77, 1.15) |
6.2 Postmarketing experience
The following adverse reactions have been identified during post-approval use of LANTUS.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure.
Medication errors have been reported in which other insulins, particularly short-acting insulins, have been accidentally administered instead of LANTUS ]. To avoid medication errors between LANTUS and other insulins, patients should be instructed to always verify the insulin label before each injection. [See Patient Counseling Information (17)
7. DRUG INTERACTIONS
A number of drugs affect glucose metabolism and may require insulin dose adjustment and particularly close monitoring.
The following are examples of drugs that may increase the blood-glucose-lowering effect of insulins including LANTUS and, therefore, increase the susceptibility to hypoglycemia: oral anti-diabetic products, pramlintide, angiotensin converting enzyme (ACE) inhibitors, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, propoxyphene, pentoxifylline, salicylates, somatostatin analogs, and sulfonamide antibiotics.
The following are examples of drugs that may reduce the blood-glucose-lowering effect of insulins including LANTUS: corticosteroids, niacin, danazol, diuretics, sympathomimetic agents (e.g., epinephrine, albuterol, terbutaline), glucagon, isoniazid, phenothiazine derivatives, somatropin, thyroid hormones, estrogens, progestogens (e.g., in oral contraceptives), protease inhibitors and atypical antipsychotic medications (e.g. olanzapine and clozapine).
Beta-blockers, clonidine, lithium salts, and alcohol may either potentiate or weaken the blood-glucose-lowering effect of insulin. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia.
The signs of hypoglycemia may be reduced or absent in patients taking sympatholytic drugs such as beta-blockers, clonidine, guanethidine, and reserpine.
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: Subcutaneous reproduction and teratology studies have been performed with insulin glargine and regular human insulin in rats and Himalayan rabbits. Insulin glargine was given to female rats before mating, during mating, and throughout pregnancy at doses up to 0.36 mg/kg/day, which is approximately 7 times the recommended human subcutaneous starting dose of 10 Units/day (0.008 mg/kg/day), based on mg/m . In rabbits, doses of 0.072 mg/kg/day, which is approximately 2 times the recommended human subcutaneous starting dose of 10 Units/day (0.008 mg/kg/day), based on mg/m , were administered during organogenesis. The effects of insulin glargine did not generally differ from those observed with regular human insulin in rats or rabbits. However, in rabbits, five fetuses from two litters of the high-dose group exhibited dilation of the cerebral ventricles. Fertility and early embryonic development appeared normal. 22
There are no well-controlled clinical studies of the use of LANTUS in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is essential for patients with diabetes or a history of gestational diabetes to maintain good metabolic control before conception and throughout pregnancy. Insulin requirements may decrease during the first trimester, generally increase during the second and third trimesters, and rapidly decline after delivery. Careful monitoring of glucose control is essential in these patients.
8.3 Nursing Mothers
It is unknown whether insulin glargine is excreted in human milk. Because many drugs, including human insulin, are excreted in human milk, caution should be exercised when LANTUS is administered to a nursing woman. Use of LANTUS is compatible with breastfeeding, but women with diabetes who are lactating may require adjustments of their insulin doses.
8.4 Pediatric Use
The safety and effectiveness of subcutaneous injections of LANTUS have been established in pediatric patients (age 6 to 15 years) with type 1 diabetes . LANTUS has not been studied in pediatric patients younger than 6 years of age with type 1 diabetes. LANTUS has not been studied in pediatric patients with type 2 diabetes. [see ] Clinical Studies (14)
Based on the results of a study in pediatric patients, the dose recommendation when switching to LANTUS is the same as that described for adults and As in adults, the dosage of LANTUS must be individualized in pediatric patients based on metabolic needs and frequent monitoring of blood glucose. [see Dosage and Administration (2.3)]. Clinical Studies (14)
8.5 Geriatric Use
In controlled clinical studies comparing LANTUS to NPH insulin, 593 of 3890 patients (15%) with type 1 and type 2 diabetes were ≥65 years of age and 80 (2%) patients were ≥75 years of age. The only difference in safety or effectiveness in the subpopulation of patients ≥65 years of age compared to the entire study population was a higher incidence of cardiovascular events typically seen in an older population in both LANTUS and NPH insulin-treated patients.
Nevertheless, caution should be exercised when LANTUS is administered to geriatric patients. In elderly patients with diabetes, the initial dosing, dose increments, and maintenance dosage should be conservative to avoid hypoglycemic reactions. Hypoglycemia may be difficult to recognize in the elderly [See ]. Warnings and Precautions (5.3)
10. OVERDOSAGE
An excess of insulin relative to food intake, energy expenditure, or both may lead to severe and sometimes prolonged and life-threatening hypoglycemia. Mild episodes of hypoglycemia can usually be treated with oral carbohydrates. Adjustments in drug dosage, meal patterns, or exercise may be needed.
More severe episodes of hypoglycemia with coma, seizure, or neurologic impairment may be treated with intramuscular/subcutaneous glucagon or concentrated intravenous glucose. After apparent clinical recovery from hypoglycemia, continued observation and additional carbohydrate intake may be necessary to avoid recurrence of hypoglycemia.
11. DESCRIPTION
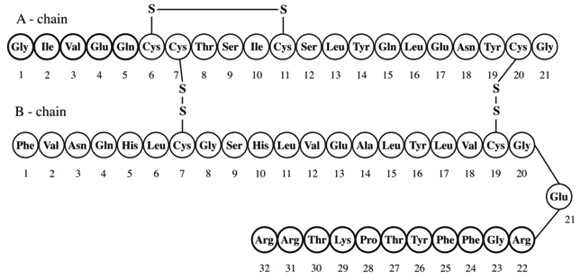
LANTUS (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as a subcutaneous injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent [ ]. LANTUS is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, insulin glargine is 21 -Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C H N O S and a molecular weight of 6063. Insulin glargine has the following structural formula: See Clinical Pharmacology (12)Escherichia coliABB26740472786
LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 Units (3.6378 mg) insulin glargine.
The 10 mL vial presentation contains the following inactive ingredients per mL: 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection.
The 3 mL cartridge presentation contains the following inactive ingredients per mL: 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection.
The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4.
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. Insulin and its analogs lower blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis and proteolysis, and enhances protein synthesis.
12.2 Pharmacodynamics
Insulin glargine is a human insulin analog that has been designed to have low aqueous solubility at neutral pH. At pH 4, as in the LANTUS injection solution, insulin glargine is completely soluble. After injection into the subcutaneous tissue, the acidic solution is neutralized, leading to formation of microprecipitates from which small amounts of insulin glargine are slowly released, resulting in a relatively constant concentration/time profile over 24 hours with no pronounced peak. This profile allows once-daily dosing as a basal insulin.
In clinical studies, the glucose-lowering effect on a molar basis (i.e., when given at the same doses) of intravenous insulin glargine is approximately the same as that for human insulin. In euglycemic clamp studies in healthy subjects or in patients with type 1 diabetes, the onset of action of subcutaneous insulin glargine was slower than NPH insulin. The effect profile of insulin glargine was relatively constant with no pronounced peak and the duration of its effect was prolonged compared to NPH insulin. shows results from a study in patients with type 1 diabetes conducted for a maximum of 24 hours after the injection. The median time between injection and the end of pharmacological effect was 14.5 hours (range: 9.5 to 19.3 hours) for NPH insulin, and 24 hours (range: 10.8 to >24.0 hours) (24 hours was the end of the observation period) for insulin glargine. Figure 1
Figure 1. Activity Profile in Patients with Type 1 Diabetes
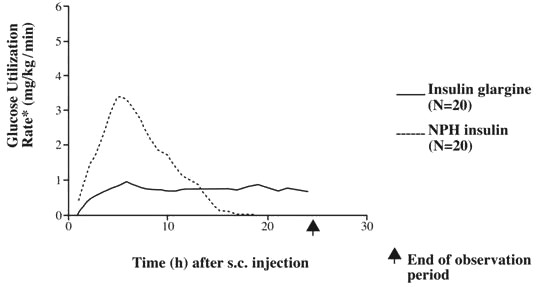
* Determined as amount of glucose infused to maintain constant plasma glucose levels (hourly mean values); indicative of insulin activity.
The longer duration of action (up to 24 hours) of LANTUS is directly related to its slower rate of absorption and supports once-daily subcutaneous administration. The time course of action of insulins, including LANTUS, may vary between individuals and within the same individual.
12.3 Pharmacokinetics
Absorption and Bioavailability. After subcutaneous injection of insulin glargine in healthy subjects and in patients with diabetes, the insulin serum concentrations indicated a slower, more prolonged absorption and a relatively constant concentration/time profile over 24 hours with no pronounced peak in comparison to NPH insulin. Serum insulin concentrations were thus consistent with the time profile of the pharmacodynamic activity of insulin glargine.
After subcutaneous injection of 0.3 Units/kg insulin glargine in patients with type 1 diabetes, a relatively constant concentration/time profile has been demonstrated. The duration of action after abdominal, deltoid, or thigh subcutaneous administration was similar.
Metabolism. A metabolism study in humans indicates that insulin glargine is partly metabolized at the carboxyl terminus of the B chain in the subcutaneous depot to form two active metabolites with in vitro activity similar to that of insulin, M1 (21 -Gly-insulin) and M2 (21 -Gly-des-30 -Thr-insulin). Unchanged drug and these degradation products are also present in the circulation. AAB
Special Populations
Information on the effect of age, race, and gender on the pharmacokinetics of LANTUS is not available. However, in controlled clinical trials in adults (n=3890) and a controlled clinical trial in pediatric patients (n=349), subgroup analyses based on age, race, and gender did not show differences in safety and efficacy between insulin glargine and NPH insulin . Age, Race, and Gender.[see ] Clinical Studies (14)
The effect of smoking on the pharmacokinetics/pharmacodynamics of LANTUS has not been studied. Smoking.
The effect of pregnancy on the pharmacokinetics and pharmacodynamics of LANTUS has not been studied [ Pregnancy.see ]. Use in Specific Populations (8.1)
. In controlled clinical trials, which included patients with Body Mass Index (BMI) up to and including 49.6 kg/m , subgroup analyses based on BMI did not show differences in safety and efficacy between insulin glargine and NPH insulin . Obesity2[see ] Clinical Studies (14)
The effect of renal impairment on the pharmacokinetics of LANTUS has not been studied. However, some studies with human insulin have shown increased circulating levels of insulin in patients with renal failure. Careful glucose monitoring and dose adjustments of insulin, including LANTUS, may be necessary in patients with renal impairment S Renal Impairment.[ee ]. Warnings and Precautions (5.5)
. The effect of hepatic impairment on the pharmacokinetics of LANTUS has not been studied. However, some studies with human insulin have shown increased circulating levels of insulin in patients with liver failure. Careful glucose monitoring and dose adjustments of insulin, including LANTUS, may be necessary in patients with hepatic impairment Hepatic Impairment[See ]. Warnings and Precautions (5.6)
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In mice and rats, standard two-year carcinogenicity studies with insulin glargine were performed at doses up to 0.455 mg/kg, which was for the rat approximately 10 times and for the mouse approximately 5 times the recommended human subcutaneous starting dose of 10 Units/day (0.008 mg/kg/day), based on mg/m . The findings in female mice were not conclusive due to excessive mortality in all dose groups during the study. Histiocytomas were found at injection sites in male rats (statistically significant) and male mice (not statistically significant) in acid vehicle containing groups. These tumors were not found in female animals, in saline control, or insulin comparator groups using a different vehicle. The relevance of these findings to humans is unknown. 2
Insulin glargine was not mutagenic in tests for detection of gene mutations in bacteria and mammalian cells (Ames- and HGPRT-test) and in tests for detection of chromosomal aberrations (cytogenetics in vitro in V79 cells and in vivo in Chinese hamsters).
In a combined fertility and prenatal and postnatal study in male and female rats at subcutaneous doses up to 0.36 mg/kg/day, which was approximately 7 times the recommended human subcutaneous starting dose of 10 Units/day (0.008 mg/kg/day), based on mg/m , maternal toxicity due to dose-dependent hypoglycemia, including some deaths, was observed. Consequently, a reduction of the rearing rate occurred in the high-dose group only. Similar effects were observed with NPH insulin. 2
14. CLINICAL STUDIES
The safety and effectiveness of LANTUS given once-daily at bedtime was compared to that of once-daily and twice-daily NPH insulin in open-label, randomized, active-controlled, parallel studies of 2,327 adult patients and 349 pediatric patients with type 1 diabetes mellitus and 1,563 adult patients with type 2 diabetes mellitus (see Tables 9–11), In general, the reduction in glycated hemoglobin (HbA1c) with LANTUS was similar to that with NPH insulin. The overall rates of hypoglycemia did not differ between patients with diabetes treated with LANTUS compared to NPH insulin . [See ] Adverse Reactions (6.1)
(see ). Type 1 Diabetes–AdultTable 11
In two clinical studies (Studies A and B), patients with type 1 diabetes (Study A; n=585, Study B; n=534) were randomized to 28 weeks of basal-bolus treatment with LANTUS or NPH insulin. Regular human insulin was administered before each meal. LANTUS was administered at bedtime. NPH insulin was administered once daily at bedtime or in the morning and at bedtime when used twice daily.
In another clinical study (Study C), patients with type 1 diabetes (n=619) were randomized to 16 weeks of basal-bolus treatment with LANTUS or NPH insulin. Insulin lispro was used before each meal. LANTUS was administered once daily at bedtime and NPH insulin was administered once or twice daily.
In these 3 studies, LANTUS and NPH insulin had similar effects on HbA1c (Table 11) with a similar overall rate of hypoglycemia . [See ] Adverse Reactions (6.1)
| Study A | Study B | Study C | ||||
|---|---|---|---|---|---|---|
| Treatment duration Treatment in combination with
| 28 weeks Regular insulin
| 28 weeks Regular insulin
| 16 weeks Insulin lispro
|
|||
| LANTUS | NPH | LANTUS | NPH | LANTUS | NPH | |
| Number of subjects treated | 292 | 293 | 264 | 270 | 310 | 309 |
| HbA1c | ||||||
| Baseline HbA1c | 8.0 | 8.0 | 7.7 | 7.7 | 7.6 | 7.7 |
| Adj. mean change from baseline | +0.2 | +0.1 | -0.2 | -0.2 | -0.1 | -0.1 |
| LANTUS – NPH | +0.1 | +0.1 | 0.0 | |||
| 95% CI for Treatment difference | (0.0; +0.2) | (-0.1; +0.2) | (-0.1; +0.1) | |||
| Basal insulin dose | ||||||
| Baseline mean | 21 | 23 | 29 | 29 | 28 | 28 |
| Mean change from baseline | -2 | 0 | -4 | +2 | -5 | +1 |
| Total insulin dose | ||||||
| Baseline mean | 48 | 52 | 50 | 51 | 50 | 50 |
| Mean change from baseline | -1 | 0 | 0 | +4 | -3 | 0 |
| Fasting blood glucose (mg/dL) | ||||||
| Baseline mean | 167 | 166 | 166 | 175 | 175 | 173 |
| Adj. mean change from baseline | -21 | -16 | -20 | -17 | -29 | -12 |
| Body weight (kg) | ||||||
| Baseline mean | 73.2 | 74.8 | 75.5 | 75.0 | 74.8 | 75.6 |
| Mean change from baseline | 0.1 | -0.0 | 0.7 | 1.0 | 0.1 | 0.5 |
Type 1 Diabetes–Pediatric (see ). Table 12
In a randomized, controlled clinical study (Study D), pediatric patients (age range 6 to 15 years) with type 1 diabetes (n=349) were treated for 28 weeks with a basal-bolus insulin regimen where regular human insulin was used before each meal. LANTUS was administered once daily at bedtime and NPH insulin was administered once or twice daily. Similar effects on HbA1c (Table 12) and the incidence of hypoglycemia were observed in both treatment groups . [See ] Adverse Reactions (6.1)
| Study D | ||
|---|---|---|
| Treatment duration | 28 weeks | |
| Treatment in combination with | Regular insulin | |
| LANTUS | NPH | |
| Number of subjects treated | 174 | 175 |
| HbA1c | ||
| Baseline mean | 8.5 | 8.8 |
| Adj. mean change from baseline | +0.3 | +0.3 |
| LANTUS – NPH | 0.0 | |
| 95% CI for Treatment difference | (-0.2; +0.3) | |
| Basal insulin dose | ||
| Baseline mean | 19 | 19 |
| Mean change from baseline | -1 | +2 |
| Total insulin dose | ||
| Baseline mean | 43 | 43 |
| Mean change from baseline | +2 | +3 |
| Fasting blood glucose (mg/dL) | ||
| Baseline mean | 194 | 191 |
| Adj. mean change from baseline | -23 | -12 |
| Body weight (kg) | ||
| Baseline mean | 45.5 | 44.6 |
| Mean change from baseline | 2.2 | 2.5 |
Type 2 Diabetes–Adult (see ). Table 13
In a randomized, controlled clinical study (Study E) (n=570), LANTUS was evaluated for 52 weeks in combination with oral anti-diabetic medications (a sulfonylurea, metformin, acarbose, or combinations of these drugs). LANTUS administered once daily at bedtime was as effective as NPH insulin administered once daily at bedtime in reducing HbA1c and fasting glucose (Table 13). The rate of hypoglycemia was similar in LANTUS and NPH insulin treated patients . [See ] Adverse Reactions (6.1)
In a randomized, controlled clinical study (Study F), in patients with type 2 diabetes not using oral anti-diabetic medications (n=518), a basal-bolus regimen of LANTUS once daily at bedtime or NPH insulin administered once or twice daily was evaluated for 28 weeks. Regular human insulin was used before meals, as needed. LANTUS had similar effectiveness as either once- or twice-daily NPH insulin in reducing HbA1c and fasting glucose (Table 13) with a similar incidence of hypoglycemia . [See ] Adverse Reactions (6.1)
In a randomized, controlled clinical study (Study G), patients with type 2 diabetes were randomized to 5 years of treatment with once-daily LANTUS or twice-daily NPH insulin. For patients not previously treated with insulin, the starting dose of LANTUS or NPH insulin was 10 units daily. Patients who were already treated with NPH insulin either continued on the same total daily NPH insulin dose or started LANTUS at a dose that was 80% of the total previous NPH insulin dose. The primary endpoint for this study was a comparison of the progression of diabetic retinopathy by 3 or more steps on the Early Treatment Diabetic Retinopathy Study (ETDRS) scale. HbA1c change from baseline was a secondary endpoint. Similar glycemic control in the 2 treatment groups was desired in order to not confound the interpretation of the retinal data. Patients or study personnel used an algorithm to adjust the LANTUS and NPH insulin doses to a target fasting plasma glucose ≤100 mg/dL. After the LANTUS or NPH insulin dose was adjusted, other anti-diabetic agents, including pre-meal insulin were to be adjusted or added. The LANTUS group had a smaller mean reduction from baseline in HbA1c compared to the NPH insulin group, which may be explained by the lower daily basal insulin doses in the LANTUS group (Table 13). Both treatment groups had a similar incidence of reported symptomatic hypoglycemia. The incidences of severe symptomatic hypoglycemia are given in Table 6 . [See ] Adverse Reactions (6.1)
| Study E | Study F | Study G | ||||
|---|---|---|---|---|---|---|
| Treatment duration | 52 weeks | 28 weeks | 5 years | |||
| Treatment in combination with | Oral agents | Regular insulin | Regular insulin | |||
| LANTUS | NPH | LANTUS | NPH | LANTUS | NPH | |
|
||||||
| Number of subjects treated | 289 | 281 | 259 | 259 | 513 | 504 |
| HbA1c | ||||||
| Baseline mean | 9.0 | 8.9 | 8.6 | 8.5 | 8.4 | 8.3 |
| Adj. mean change from baseline | -0.5 | -0.4 | -0.4 | -0.6 | -0.6 | -0.8 |
| LANTUS – NPH | -0.1 | +0.2 | +0.2 | |||
| 95% CI for Treatment difference | (-0.3; +0.1) | (0.0; +0.4) | (+0.1, +0.4) | |||
| Basal insulin dose * | ||||||
| Baseline mean | 14 | 15 | 44.1 | 45.5 | 39 | 44 |
| Mean change from baseline | +12 | +9 | -1 | +7 | +23 | +30 |
| Total insulin dose * | ||||||
| Baseline mean | 14 | 15 | 64 | 67 | 48 | 53 |
| Mean change from baseline | +12 | +9 | +10 | +13 | +41 | +40 |
| Fasting blood glucose (mg/dL) | ||||||
| Baseline mean | 179 | 180 | 164 | 166 | 190 | 180 |
| Adj. mean change from baseline | -49 | -46 | -24 | -22 | -45 | -44 |
| Body weight (kg) | ||||||
| Baseline mean | 83.5 | 82.1 | 89.6 | 90.7 | 100 | 99 |
| Adj. mean change from baseline | 2.0 | 1.9 | 0.4 | 1.4 | 3.7 | 4.8 |
LANTUS Timing of Daily Dosing (see ). Table 14
The safety and efficacy of LANTUS administered pre-breakfast, pre-dinner, or at bedtime were evaluated in a randomized, controlled clinical study in patients with type 1 diabetes (study H, n=378). Patients were also treated with insulin lispro at mealtime. LANTUS administered at different times of the day resulted in similar reductions in HbA1c compared to that with bedtime administration (see ). In these patients, data are available from 8-point home glucose monitoring. The maximum mean blood glucose was observed just prior to injection of LANTUS regardless of time of administration. Table 14
In this study, 5% of patients in the LANTUS-breakfast arm discontinued treatment because of lack of efficacy. No patients in the other two arms discontinued for this reason. The safety and efficacy of LANTUS administered pre-breakfast or at bedtime were also evaluated in a randomized, active-controlled clinical study (Study I, n=697) in patients with type 2 diabetes not adequately controlled on oral anti-diabetic therapy. All patients in this study also received glimepiride 3 mg daily. LANTUS given before breakfast was at least as effective in lowering HbA1c as LANTUS given at bedtime or NPH insulin given at bedtime (see ). Table 14
| Study H | Study I | |||||
|---|---|---|---|---|---|---|
| Treatment duration | 24 weeks | 24 weeks | ||||
| Treatment in combination with: | Insulin lispro | Glimepiride | ||||
| LANTUS Breakfast
| LANTUS Dinner
| LANTUS Bedtime
| LANTUS Breakfast
| LANTUS Bedtime
| NPH Bedtime
|
|
| **total number of patients evaluable for safety | ||||||
| Number of subjects treated * | 112 | 124 | 128 | 234 | 226 | 227 |
| HbA1c | ||||||
| Baseline mean | 7.6 | 7.5 | 7.6 | 9.1 | 9.1 | 9.1 |
| Mean change from baseline | -0.2 | -0.1 | 0.0 | -1.3 | -1.0 | -0.8 |
| Basal insulin dose (U) | ||||||
| Baseline mean | 22 | 23 | 21 | 19 | 20 | 19 |
| Mean change from baseline | 5 | 2 | 2 | 11 | 18 | 18 |
| Total insulin dose (U) | NA † | NA | NA | |||
| Baseline mean | 52 | 52 | 49 | |||
| Mean change from baseline | 2 | 3 | 2 | |||
| Body weight (kg) | ||||||
| Baseline mean | 77.1 | 77.8 | 74.5 | 80.7 | 82 | 81 |
| Mean change from baseline | 0.7 | 0.1 | 0.4 | 3.9 | 3.7 | 2.9 |
16. HOW SUPPLIED/STORAGE AND HANDLING
NDC:64725-2220-1 in a VIAL, GLASS of 10 INJECTION, SOLUTIONS
16.1 How supplied
LANTUS solution for injection 100 units per mL (U-100) is available as:
| Dosage Unit/Strength | Package size | NDC # 0088
|
|---|---|---|
| 100 Units/mL
10 mL vials
| Pack of 1 | 2220-33 |
| 100 Units/mL
3 mL SoloStar disposable insulin device
®
| package of 5 | 2219-05 |
Needles are not included in the packs.
BD Ultra-Fine™ needles to be used in conjunction with SoloStar are sold separately and are manufactured by BD. 1
- 1
- The brands listed are the registered trademarks of their respective owners and are not trademarks of sanofi-aventis U.S. LLC
16.2 Storage
LANTUS should not be stored in the freezer and should not be allowed to freeze. Discard LANTUS if it has been frozen.
Unopened Vial/ SoloStar disposable insulin device:
Unopened LANTUS vials, cartridge systems and SoloStar device should be stored in a refrigerator, 36°F – 46°F (2°C – 8°C). Discard after the expiration date.
Open (In-Use) Vial:
Vials must be discarded 28 days after being opened. If refrigeration is not possible, the open vial can be kept unrefrigerated for up to 28 days away from direct heat and light, as long as the temperature is not greater than 86°F (30°C).
Open (In-Use) SoloStar disposable insulin device:
The opened (in-use) SoloStar should NOT be refrigerated but should be kept at room temperature (below 86°F [30°C]) away from direct heat and light. The opened (in-use) SoloStar device must be discarded 28 days after being opened.
These storage conditions are summarized in the following table:
| Not in-use (unopened) Refrigerated
| Not in-use (unopened) Room Temperature
| In-use (opened) (See Temperature Below)
|
|
|---|---|---|---|
| 10 mL Vial | Until expiration date | 28 days | 28 days Refrigerated or room temperature
|
| 3 mL SoloStar disposable insulin device ® | Until expiration date | 28 days | 28 days Room temperature only (Do not refrigerate)
|
16.3 Preparation and handling
Parenteral drug products should be inspected visually prior to administration whenever the solution and the container permit. LANTUS must only be used if the solution is clear and colorless with no particles visible.
Mixing and diluting: LANTUS must NOT be diluted or mixed with any other insulin or solution [S . ee ] Warnings and Precautions (5.2)
The syringes must not contain any other medicinal product or residue. Vial:
: If SoloStar disposable insulin device, malfunctions, LANTUS may be drawn from the cartridge system or from SoloStar into a U-100 syringe and injected. SoloStar
17. PATIENT COUNSELING INFORMATION
17.1 Instructions for patients
Patients should be informed that changes to insulin regimens must be made cautiously and only under medical supervision.
Patients should be informed about the potential side effects of insulin therapy, including lipodystrophy (and the need to rotate injection sites within the same body region), weight gain, allergic reactions, and hypoglycemia. Patients should be informed that the ability to concentrate and react may be impaired as a result of hypoglycemia. This may present a risk in situations where these abilities are especially important, such as driving or operating other machinery. Patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia should be advised to use caution when driving or operating machinery.
Accidental mix-ups between LANTUS and other insulins, particularly short-acting insulins, have been reported. To avoid medication errors between LANTUS and other insulins, patients should be instructed to always check the insulin label before each injection.
LANTUS must only be used if the solution is clear and colorless with no particles visible Patients must be advised that LANTUS must NOT be diluted or mixed with any other insulin or solution ..
Patients should be advised not to share disposable or reusable insulin devices or needles with other patients, because doing so carries a risk for transmission of blood-borne pathogens.
Patients should be instructed on self-management procedures including glucose monitoring, proper injection technique, and management of hypoglycemia and hyperglycemia. Patients must be instructed on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, and skipped meals.
Patients with diabetes should be advised to inform their health care professional if they are pregnant or are contemplating pregnancy.
Refer patients to the LANTUS "Patient Information" for additional information.
Patient Information LANTUS 10 mL vial (1000 units per vial) 100 units per mL (U-100) (insulin glargine [recombinant DNA origin] injection)
®
- What is the most important information I should know about LANTUS?
- What is LANTUS?
- Who should NOT take LANTUS?
- How should I use LANTUS?
- What kind of syringe should I use?
- Mixing with LANTUS
- Instructions for Use
- How do I draw the insulin into the syringe?
- How do I inject LANTUS?
- What can affect how much insulin I need?
- What are the possible side effects of LANTUS and other insulins?
- How should I store LANTUS?
- General Information about LANTUS
Read this "Patient Information" that comes with LANTUS (LAN-tus) before you start using it and each time you get a refill because there may be new information. This leaflet does not take the place of talking with your healthcare provider about your condition or treatment. If you have questions about LANTUS or about diabetes, talk with your healthcare provider.
What is the most important information I should know about LANTUS?
- Any change of insulin should be made cautiously and only under medical supervision. Changes in insulin strength, manufacturer, type (for example: Regular, NPH, analogs), species (beef, pork, beef-pork, human) or method of manufacture (recombinant DNA versus animal source insulin) may need a change in the dose. This dose change may be needed right away or later on during the first several weeks or months on the new insulin. Doses of oral anti-diabetic medicines may also need to change, if your insulin is changed. Do not change the insulin you are using without talking to your healthcare provider.
- Your healthcare provider will tell you how often you should test your blood sugar level, and what to do if it is high or low. You must test your blood sugar levels while using an insulin, such as LANTUS.
- It will not work and you may lose blood sugar control, which could be serious. Do NOT dilute or mix LANTUS with any other insulin or solution.
- comes as U-100 insulin and contains 100 units of LANTUS per milliliter (mL). One milliliter of U-100 insulin contains 100 units of insulin. (1 mL = 1 cc). LANTUS
What is Diabetes?
- Your body needs insulin to turn sugar (glucose) into energy. If your body does not make enough insulin, you need to take more insulin so you will not have too much sugar in your blood.
- Insulin injections are important in keeping your diabetes under control. But the way you live, your diet, careful checking of your blood sugar levels, exercise, and planned physical activity, all work with your insulin to help you control your diabetes.
What is LANTUS?
- LANTUS (insulin glargine [recombinant DNA origin]) is a long-acting insulin. Because LANTUS is made by recombinant DNA technology (rDNA) and is chemically different from the insulin made by the human body, it is called an insulin analog. LANTUS is used to treat patients with diabetes for the control of high blood sugar. It is used once a day to lower blood sugar.
- LANTUS is a clear, colorless, sterile solution for injection under the skin (subcutaneously).
- The active ingredient in LANTUS is insulin glargine. The concentration of insulin glargine is 100 units per milliliter (mL), or U-100. LANTUS also contains zinc, metacresol, glycerol, polysorbate 20 and water for injection as inactive ingredients. Hydrochloric acid and/or sodium hydroxide may be added to adjust the pH.
- You need a prescription to get LANTUS. Always be sure you receive the right insulin from the pharmacy.
Who should NOT take LANTUS?
or any of the inactive ingredients in LANTUS. Check with your healthcare provider if you are not sure. Do not take LANTUS if you are allergic to insulin glargine
Before starting LANTUS, tell your healthcare provider about all your medical conditions including if you:
- Your dose may need to be adjusted. have liver or kidney problems.
- It is not known if LANTUS may harm your unborn baby. It is very important to maintain control of your blood sugar levels during pregnancy. Your healthcare provider will decide which insulin is best for you during your pregnancy. are pregnant or plan to become pregnant.
- It is not known whether LANTUS passes into your milk. Many medicines, including insulin, pass into human milk, and could affect your baby. Talk to your healthcare provider about the best way to feed your baby. are breast-feeding or plan to breast-feed.
- prescription and non-prescription medicines, vitamins, and herbal supplements. about all the medicines you take including
- , especially ones commonly called TZDs (thiazolidinediones). if you take any other medicines
- . If you have heart failure, it may get worse while you take TZDs with Lantus. if you have heart failure or other heart problems
How should I use LANTUS?
See the including the "Instructions for Use""How do I draw the insulin into the syringe?" section for additional information.
- Follow the instructions given by your healthcare provider about the type or types of insulin you are using. Do not make any changes with your insulin unless you have talked to your healthcare provider. Your insulin needs may change because of illness, stress, other medicines, or changes in diet or activity level. Talk to your healthcare provider about how to adjust your insulin dose.
- You may take LANTUS at any time during the day but you must take it at the same time every day.
- Only use LANTUS that is clear and colorless. If your LANTUS is cloudy or slightly colored, return it to your pharmacy for a replacement.
- Follow your healthcare provider's instructions for testing your blood sugar.
- Inject LANTUS under your skin (subcutaneously) in your upper arm, abdomen (stomach area), or thigh (upper leg). Never inject it into a vein or muscle.
- Change (rotate) injection sites within the same body area.
What kind of syringe should I use?
- Always use a syringe that is marked for U-100 insulin. If you use other than U-100 insulin syringe, you may get the wrong dose of insulin causing serious problems for you, such as a blood sugar level that is too low or too high. Always use a new needle and syringe each time you give LANTUS injection.
- NEEDLES AND SYRINGES MUST NOT BE SHARED.
- Disposable syringes and needles should be used only once. Used syringe and needle should be placed in sharps containers (such as red biohazard containers), hard plastic containers (such as detergent bottles), or metal containers (such as an empty coffee can). Such containers should be sealed and disposed of properly.
Mixing with LANTUS
- It will not work as intended and you may lose blood sugar control, which could be serious. Do NOT dilute or mix LANTUS with any other insulin or solution.
Instructions for Use
How do I draw the insulin into the syringe?
- The syringe must be new and does not contain any other medicine.
- Do not mix LANTUS with any other type of insulin.
Follow these steps:
- Wash your hands with soap and water or with alcohol.
- Check the insulin to make sure it is clear and colorless. Do not use the insulin after the expiration date stamped on the label, if it is colored or cloudy, or if you see particles in the solution.
- If you are using a new vial, remove the protective cap. remove the stopper.
Do not
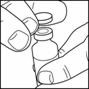
- Wipe the top of the vial with an alcohol swab. You do not have to shake the vial of LANTUS before use.

- Use a new needle and syringe every time you give an injection. Use disposable syringes and needles only once. Throw them away properly. share needles and syringes. Never
- Draw air into the syringe equal to your insulin dose. Put the needle through the rubber top of the vial and push the plunger to inject the air into the vial.
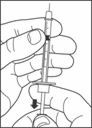
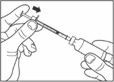
- Leave the syringe in the vial and turn both upside down. Hold the syringe and vial firmly in one hand.
- Make sure the tip of the needle is in the insulin. With your free hand, pull the plunger to withdraw the correct dose into the syringe.
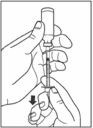
- Before you take the needle out of the vial, check the syringe for air bubbles. If bubbles are in the syringe, hold the syringe straight up and tap the side of the syringe until the bubbles float to the top. Push the bubbles out with the plunger and draw insulin back in until you have the correct dose.
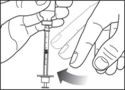
- Remove the needle from the vial. Do not let the needle touch anything. You are now ready to inject.
How do I inject LANTUS?
Inject LANTUS under your skin. Take LANTUS as prescribed by your healthcare provider.
Follow these steps:
- Decide on an injection area - either upper arm, thigh or abdomen. Injection sites within an injection area must be different from one injection to the next.
- Use alcohol or soap and water to clean the injection site. The injection site should be dry before you inject.
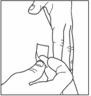
- Pinch the skin. Stick the needle in the way your healthcare provider showed you. Release the skin.
- Slowly push in the plunger of the syringe all the way, making sure you have injected all the insulin. Leave the needle in the skin for about 10 seconds.
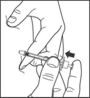
- Pull the needle straight out and gently press on the spot where you injected yourself for several seconds. Do not rub the area.
- Follow your healthcare provider's instructions for throwing away the used needle and syringe. Do not recap the used needle. Used needle and syringe should be placed in sharps containers (such as red biohazard containers), hard plastic containers (such as detergent bottles), or metal containers (such as an empty coffee can). Such containers should be sealed and disposed of properly.
What can affect how much insulin I need?
Illness may change how much insulin you need. It is a good idea to think ahead and make a "sick day" plan with your healthcare provider in advance so you will be ready when this happens. Be sure to test your blood sugar more often and call your healthcare provider if you are sick. Illness.
Other medicines, including prescription and non-prescription medicines, vitamins, and herbal supplements, can change the way insulin works. You may need a different dose of insulin when you are taking certain other medicines. including prescription and non-prescription medicines, vitamins, and herbal supplements. You may want to keep a list of the medicines you take. You can show this list to your healthcare provider anytime you get a new medicine or refill. Your healthcare provider will tell you if your insulin dose needs to be changed. Medicines. Many medicines can affect your insulin needs.Know all the medicines you take,
The amount of food you eat can affect your insulin needs. If you eat less food, skip meals, or eat more food than usual, you may need a different dose of insulin. Talk to your healthcare provider if you change your diet so that you know how to adjust your LANTUS and other insulin doses. Meals.
Alcohol, including beer and wine, may affect the way LANTUS works and affect your blood sugar levels. Talk to your healthcare provider about drinking alcohol. Alcohol.
Exercise or activity level may change the way your body uses insulin. Check with your healthcare provider before you start an exercise program because your dose may need to be changed. Exercise or Activity level.
If you travel across time zones, talk with your healthcare provider about how to time your injections. When you travel, wear your medical alert identification. Take extra insulin and supplies with you. Travel.
The effects of LANTUS on an unborn child or on a nursing baby are unknown. Therefore, tell your healthcare provider if you are planning to have a baby, are pregnant, or nursing a baby. Good control of diabetes is especially important during pregnancy and nursing. Pregnancy or nursing.
What are the possible side effects of LANTUS and other insulins?
Insulins, including LANTUS, can cause hypoglycemia (low blood sugar), hyperglycemia (high blood sugar), allergy, and skin reactions.
Hypoglycemia (low blood sugar):
Hypoglycemia is often called an "insulin reaction" or "low blood sugar". It may happen when you do not have enough sugar in your blood. Common causes of hypoglycemia are illness, emotional or physical stress, too much insulin, too little food or missed meals, and too much exercise or activity.
Early warning signs of hypoglycemia may be different, less noticeable or not noticeable at all in some people. That is why it is important to check your blood sugar as you have been advised by your healthcare provider.
: Hypoglycemia can happen with
- This can happen when too much insulin is injected. Taking too much insulin.
- . This can happen if a meal or snack is missed or delayed. Not enough carbohydrate (sugar or starch) intake
- that decreases the amount of sugar absorbed by your body. Vomiting or diarrhea
- Intake of alcohol.
- Be sure to discuss all your medicines with your healthcare provider. Medicines that affect insulin.Do not start any new medicines until you know how they may affect your insulin dose.
- These conditions include diseases of the adrenal glands, the pituitary, the thyroid gland, the liver, and the kidney. Medical conditions that can affect your blood sugar levels or insulin.
- This can happen if you exercise too much or have a fever. Too much glucose use by the body.
- Injecting insulin the wrong way or in the wrong injection area.
Hypoglycemia can be mild to severe. Its onset may be rapid. Some patients have few or no warning symptoms, including:
- patients with diabetes for a long time
- patients with diabetic neuropathy (nerve problems)
- or patients using certain medicines for high blood pressure or heart problems.
Hypoglycemia may reduce your ability to drive a car or use mechanical equipment and you may risk injury to yourself or others.
Severe hypoglycemia can be dangerous and can cause temporary or permanent harm to your heart or brain. It may cause unconsciousness, seizures, or death.
Symptoms of hypoglycemia may include:
- anxiety, irritability, restlessness, trouble concentrating, personality changes, mood changes, or other abnormal behavior
- tingling in your hands, feet, lips, or tongue
- dizziness, light-headedness, or drowsiness
- nightmares or trouble sleeping
- headache
- blurred vision
- slurred speech
- palpitations (fast heart beat)
- sweating
- tremor (shaking)
- unsteady gait (walking).
If you have hypoglycemia often or it is hard for you to know if you have the symptoms of hypoglycemia, talk to your healthcare provider.
Mild to moderate hypoglycemia is treated by eating or drinking carbohydrates, such as fruit juice, raisins, sugar candies, milk or glucose tablets. Talk to your healthcare provider about the amount of carbohydrates you should eat to treat mild to moderate hypoglycemia.
Severe hypoglycemia may require the help of another person or emergency medical people. A person with hypoglycemia who is unable to take foods or liquids with sugar by mouth, or is unconscious needs medical help fast and will need treatment with a glucagon injection or glucose given intravenously (IV). Without medical help right away, serious reactions or even death could happen.
Hyperglycemia (high blood sugar):
Hyperglycemia happens when you have too much sugar in your blood. Usually, it means there is not enough insulin to break down the food you eat into energy your body can use. Hyperglycemia can be caused by a fever, an infection, stress, eating more than you should, taking less insulin than prescribed, or it can mean your diabetes is getting worse.
Hyperglycemia can happen with:
- . This can happen from:
Insufficient (too little) insulin
- -
- injecting too little or no insulin
- -
- incorrect storage (freezing, excessive heat)
- -
- use after the expiration date.
- . This can happen if you eat larger meals, eat more often, or increase the amount of carbohydrate in your meals. Too much carbohydrate intake
- Be sure to discuss all your medicines with your healthcare provider. Medicines that affect insulin.Do not start any new medicines until you know how they may affect your insulin dose.
- . These medical conditions include fevers, infections, heart attacks, and stress. Medical conditions that affect insulin
- Injecting insulin the wrong way or in the wrong injection area.
Testing your blood or urine often will let you know if you have hyperglycemia. If your tests are often high, tell your healthcare provider so your dose of insulin can be changed.
Hyperglycemia can be mild or severe. Hyperglycemia can progress to diabetic ketoacidosis (DKA) or very high glucose levels (hyperosmolar coma) and result in unconsciousness and death.
Although diabetic ketoacidosis occurs most often in patients with type 1 diabetes, it can also happen in patients with type 2 diabetes who become very sick. Because some patients get few symptoms of hyperglycemia, it is important to check your blood sugar/urine sugar and ketones regularly.
Symptoms of hyperglycemia include:
- confusion or drowsiness
- increased thirst
- decreased appetite, nausea, or vomiting
- rapid heart rate
- increased urination and dehydration (too little fluid in your body).
Symptoms of DKA also include:
- fruity smelling breath
- fast, deep breathing
- stomach area (abdominal) pain.
Severe or continuing hyperglycemia or DKA needs evaluation and treatment right away by your healthcare provider.
Do not use LANTUS to treat diabetic ketoacidosis.
Other possible side effects of LANTUS include:
Serious allergic reactions:
Some times severe, life-threatening allergic reactions can happen with insulin. If you think you are having a severe allergic reaction, get medical help right away. Signs of insulin allergy include:
- rash all over your body
- shortness of breath
- wheezing (trouble breathing)
- fast pulse
- sweating
- low blood pressure.
Reactions at the injection site:
Injecting insulin can cause the following reactions on the skin at the injection site:
- little depression in the skin (lipoatrophy)
- skin thickening (lipohypertrophy)
- red, swelling, itchy skin (injection site reaction).
You can reduce the chance of getting an injection site reaction if you change (rotate) the injection site each time. An injection site reaction should clear up in a few days or a few weeks. If injection site reactions do not go away or keep happening, call your healthcare provider.
Swelling of your hands and feet (edema)
Weight gain
Taking certain diabetes pills called thiazolidinediones or "TZDs" with Lantus may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with Lantus. Your healthcare provider should monitor you closely while you are taking TZDs with Lantus. Tell your healthcare provider if you have any new or worse symptoms of heart failure including: Heart Failure.
- shortness of breath
- swelling of your ankles or feet
- sudden weight gain
During treatment with TZDs and Lantus, the TZD dose may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
Tell your healthcare provider if you have any side effects that bother you.
These are not all the side effects of LANTUS. Ask your healthcare provider or pharmacist for more information.
How should I store LANTUS?
-
Unopened vial:
Store new (unopened) LANTUS vials in a refrigerator (not the freezer) between 36°F to 46°F (2°C to 8°C). Do not freeze LANTUS. Keep LANTUS out of direct heat and light. If a vial has been frozen or overheated, throw it away.
- :
Open (In-Use) vial
Once a vial is opened, you can keep it in a refrigerator or at room temperature (below 86°F [30°C]) but away from direct heat and light. Opened vial, either kept in a refrigerator or at room temperature, should be discarded 28 days after the first use even if it still contains LANTUS. Do not leave your insulin in a car on a summer day.
These storage conditions are summarized in the following table:
Not in-use (unopened)
Not in-use (unopened)
In-use (opened)
Refrigerated Room Temperature
(See Temperature Below) 10 mL Vial Until expiration date 28 days 28 days Refrigerated or room temperature
- Do not use a vial of LANTUS after the expiration date stamped on the label.
- Do not use LANTUS if it is cloudy, colored, or if you see particles.
General Information about LANTUS
- Use LANTUS only to treat your diabetes. give or share LANTUS with another person, even if they have diabetes also. It may harm them. Do not
- This leaflet summarizes the most important information about LANTUS. If you would like more information, talk with your healthcare provider. You can ask your doctor or pharmacist for information about LANTUS that is written for healthcare professionals. For more information about LANTUS call 1-800-633-1610 or go to website www.lantus.com.
ADDITIONAL INFORMATION
is a national magazine designed especially for patients with diabetes and their families and is available by subscription from the American Diabetes Association (ADA), P.O.Box 363, Mt. Morris, IL 61054-0363, 1-800-DIABETES (1-800-342-2383). You may also visit the ADA website at www.diabetes.org. DIABETES FORECAST
Another publication, , is available from the Juvenile Diabetes Research Foundation International (JDRF), 120 Wall Street, 19th Floor, New York, New York 10005, 1-800-JDF-CURE (1-800-533-2873). You may also visit the JDRF website at www.jdf.org. COUNTDOWN
To get more information about diabetes, check with your healthcare professional or diabetes educator or visit www.DiabetesWatch.com.
Additional information about LANTUS can be obtained by calling 1-800-633-1610 or by visiting www.lantus.com.
Rev. October 2013
sanofi-aventis U.S. LLC Bridgewater, NJ 08807 A SANOFI COMPANY
©2013 sanofi-aventis U.S. LLC
Patient Information LANTUS SOLOSTAR 3 mL disposable insulin delivery device (300 units per device) 100 units per mL (U-100) (insulin glargine [recombinant DNA origin] injection)
®®
- What is the most important information I should know about LANTUS?
- What is LANTUS?
- Who should NOT take LANTUS?
- How should I use LANTUS?
- Mixing with LANTUS
- Instructions for Use
- What can affect how much insulin I need?
- What are the possible side effects of LANTUS and other insulins?
- How should I store LANTUS?
- General Information about LANTUS
Read this "Patient Information" that comes with LANTUS (LAN-tus) before you start using it and each time you get a refill because there may be new information. This leaflet does not take the place of talking with your healthcare provider about your condition or treatment. If you have questions about LANTUS or about diabetes, talk with your healthcare provider.
What is the most important information I should know about LANTUS?
- Any change of insulin should be made cautiously and only under medical supervision. Changes in insulin strength, manufacturer, type (for example: Regular, NPH, analogs), species (beef, pork, beef-pork, human) or method of manufacture (recombinant DNA versus animal-source insulin) may need a change in the dose. This dose change may be needed right away or later on during the first several weeks or months on the new insulin. Doses of oral anti-diabetic medicines may also need to change, if your insulin is changed. Do not change the insulin you are using without talking to your healthcare provider.
- Your healthcare provider will tell you how often you should test your blood sugar level, and what to do if it is high or low. You must test your blood sugar levels while using an insulin, such as LANTUS.
- It will not work and you may lose blood sugar control, which could be serious. Do NOT dilute or mix LANTUS with any other insulin or solution.
- comes as U-100 insulin and contains 100 units of LANTUS per milliliter (mL). One milliliter of U-100 insulin contains 100 units of insulin. (1 mL = 1 cc). LANTUS
What is Diabetes?
- Your body needs insulin to turn sugar (glucose) into energy. If your body does not make enough insulin, you need to take more insulin so you will not have too much sugar in your blood.
- Insulin injections are important in keeping your diabetes under control. But the way you live, your diet, careful checking of your blood sugar levels, exercise, and planned physical activity, all work with your insulin to help you control your diabetes.
What is LANTUS?
- LANTUS (insulin glargine [recombinant DNA origin]) is a long-acting insulin. Because Lantus is made by recombinant DNA technology (rDNA) and is chemically different from the insulin made by the human body, it is called an insulin analog. LANTUS is used to treat patients with diabetes for the control of high blood sugar. It is used once a day to lower blood glucose.
- LANTUS is a clear, colorless, sterile solution for injection under the skin (subcutaneously).
- The active ingredient in LANTUS is insulin glargine. The concentration of insulin glargine is 100 units per milliliter (mL), or U-100. LANTUS also contains zinc, metacresol, glycerol, and water for injection as inactive ingredients. Hydrochloric acid and/or sodium hydroxide may be added to adjust the pH.
- You need a prescription to get LANTUS. Always be sure you receive the right insulin from the pharmacy.
Who should NOT take LANTUS?
or any of the inactive ingredients in LANTUS. Check with your healthcare provider if you are not sure. Do not take LANTUS if you are allergic to insulin glargine
-
Before starting LANTUS, tell your healthcare provider about all your medical conditions including if you:
- Your dose may need to be adjusted. have liver or kidney problems.
- It is not known if LANTUS may harm your unborn baby. It is very important to maintain control of your blood sugar levels during pregnancy. Your healthcare provider will decide which insulin is best for you during your pregnancy. are pregnant or plan to become pregnant.
- It is not known whether LANTUS passes into your milk. Many medicines, including insulin, pass into human milk, and could affect your baby. Talk to your healthcare provider about the best way to feed your baby. are breast-feeding or plan to breast-feed.
- prescription and non-prescription medicines, vitamins and herbal supplements. are taking any other medicines including
- , especially ones commonly called TZDs (thiazolidinediones). if you take any other medicines
- . If you have heart failure, it may get worse while you take TZDs with Lantus. if you have heart failure or other heart problems
How should I use LANTUS?
See the " Instructions for SoloStar Use" section for additional information.
- Follow the instructions given by your healthcare provider about the type or types of insulin you are using. Do not make any changes with your insulin unless you have talked to your healthcare provider. Your insulin needs may change because of illness, stress, other medicines, or changes in diet or activity level. Talk to your healthcare provider about how to adjust your insulin dose.
- You may take LANTUS at any time during the day but you must take it at the same time every day.
- Only use LANTUS that is clear and colorless. If your LANTUS is cloudy or slightly colored, return it to your pharmacy for a replacement.
- Follow your healthcare provider's instructions for testing your blood sugar.
- Inject LANTUS under your skin (subcutaneously) in your upper arm, abdomen (stomach area), or thigh (upper leg). Never inject it into a vein or muscle.
- Change (rotate) injection sites within the same body area.
-
NEEDLES AND SOLOSTAR MUST NOT BE SHARED.
- Disposable needles should be used only once. Used needle should be placed in sharps containers (such as red biohazard containers), hard plastic containers (such as detergent bottles), or metal containers (such as an empty coffee can). Such containers should be sealed and disposed of properly.
Mixing with LANTUS
- It will not work as intended and you may lose blood sugar control, which could be serious. Do NOT dilute or mix LANTUS with any other insulin or solution.
Instructions for SoloStar Use
If you have lost your leaflet or have a question, go to www.lantus.com or call 1-800-633-1610. It is important to read, understand, and follow the step-by-step instructions in the "SoloStar Instruction Leaflet" before using SoloStar disposable insulin Pen. Failure to follow the instructions may result in getting too much or too little insulin.
The following general notes should be taken into consideration before injecting Lantus:
- Always wash your hands before handling the SoloStar disposable insulin Pen.
- Always attach a new needle before use. BD Ultra-Fine™ needles are compatible with SoloStar. These are sold separately and are manufactured by BD. †
- Always perform the safety test before use.
- Check the insulin solution in the pen to make sure it is clear, colorless, and free of particles. If it is not, throw it away.
- Do NOT mix or dilute LANTUS with any other insulin or solution. LANTUS will not work if it is mixed or diluted and you may lose blood sugar control, which could be serious.
- Decide on an injection area - either upper arm, thigh, or abdomen. Do not use the same injection site as your last injection.
- After injecting LANTUS, leave the needle in the skin for an additional 10 seconds. Then pull the needle straight out. Gently press on the spot where you injected yourself for a few seconds. Do not rub the area.
- Do not drop the SoloStar disposable insulin Pen.
If your blood glucose reading is high or low, tell your healthcare provider so the dose can be adjusted.
What can affect how much insulin I need?
Illness may change how much insulin you need. It is a good idea to think ahead and make a "sick day" plan with your healthcare provider in advance so you will be ready when this happens. Be sure to test your blood sugar more often and call your healthcare provider if you are sick. Illness.
Other medicines, including prescription and non-prescription medicines, vitamins, and herbal supplements, can change the way insulin works. You may need a different dose of insulin when you are taking certain other medicines. including prescription and non-prescription medicines, vitamins and herbal supplements. You may want to keep a list of the medicines you take. You can show this list to your healthcare provider and pharmacists anytime you get a new medicine or refill. Your healthcare provider will tell you if your insulin dose needs to be changed. Medicines. Many medicines can affect your insulin needs.Know all the medicines you take,
The amount of food you eat can affect your insulin needs. If you eat less food, skip meals, or eat more food than usual, you may need a different dose of insulin. Talk to your healthcare provider if you change your diet so that you know how to adjust your LANTUS and other insulin doses. Meals.
Alcohol, including beer and wine, may affect the way LANTUS works and affect your blood sugar levels. Talk to your healthcare provider about drinking alcohol. Alcohol.
Exercise or activity level may change the way your body uses insulin. Check with your healthcare provider before you start an exercise program because your dose may need to be changed. Exercise or Activity level.
If you travel across time zones, talk with your healthcare provider about how to time your injections. When you travel, wear your medical alert identification. Take extra insulin and supplies with you. Travel.
The effects of LANTUS on an unborn child or on a nursing baby are unknown. Therefore, tell your healthcare provider if you are planning to have a baby, are pregnant, or nursing a baby. Good control of diabetes is especially important during pregnancy and nursing. Pregnancy or nursing.
What are the possible side effects of LANTUS and other insulins?
Insulins, including LANTUS, can cause hypoglycemia (low blood sugar), hyperglycemia (high blood sugar), allergy, and skin reactions.
Hypoglycemia (low blood sugar):
Hypoglycemia is often called an "insulin reaction" or "low blood sugar". It may happen when you do not have enough sugar in your blood. Common causes of hypoglycemia are illness, emotional or physical stress, too much insulin, too little food or missed meals, and too much exercise or activity.
Early warning signs of hypoglycemia may be different, less noticeable or not noticeable at all in some people. That is why it is important to check your blood sugar as you have been advised by your healthcare provider.
Hypoglycemia can happen with:
- This can happen when too much insulin is injected. Taking too much insulin.
- This can happen if a meal or snack is missed or delayed. Not enough carbohydrate (sugar or starch) intake.
- that decreases the amount of sugar absorbed by your body. Vomiting or diarrhea
- Intake of alcohol.
- Be sure to discuss all your medicines with your healthcare provider. Medicines that affect insulin.Do not start any new medicines until you know how they may affect your insulin dose.
- These conditions include diseases of the adrenal glands, the pituitary, the thyroid gland, the liver, and the kidney. Medical conditions that can affect your blood sugar levels or insulin.
- This can happen if you exercise too much or have a fever. Too much glucose use by the body.
- Injecting insulin the wrong way or in the wrong injection area.
Hypoglycemia can be mild to severe. Its onset may be rapid. Some patients have few or no warning symptoms, including:
- patients with diabetes for a long time
- patients with diabetic neuropathy (nerve problems)
- or patients using certain medicines for high blood pressure or heart problems.
Hypoglycemia may reduce your ability to drive a car or use mechanical equipment and you may risk injury to yourself or others.
Severe hypoglycemia can be dangerous and can cause temporary or permanent harm to your heart or brain. It may cause unconsciousness, seizures, or death.
Symptoms of hypoglycemia may include:
- anxiety, irritability, restlessness, trouble concentrating, personality changes, mood changes, or other abnormal behavior
- tingling in your hands, feet, lips, or tongue
- dizziness, light-headedness, or drowsiness
- nightmares or trouble sleeping
- headache
- blurred vision
- slurred speech
- palpitations (fast heart beat)
- sweating
- tremor (shaking)
- unsteady gait (walking).
If you have hypoglycemia often or it is hard for you to know if you have the symptoms of hypoglycemia, talk to your healthcare provider.
Mild to moderate hypoglycemia is treated by eating or drinking carbohydrates such as fruit juice, raisins, sugar candies, milk or glucose tablets. Talk to your healthcare provider about the amount of carbohydrates you should eat to treat mild to moderate hypoglycemia.
Severe hypoglycemia may require the help of another person or emergency medical people. A person with hypoglycemia who is unable to take foods or liquids with sugar by mouth, or is unconscious needs medical help fast and will need treatment with a glucagon injection or glucose given intravenously (IV). Without medical help right away, serious reactions or even death could happen.
Hyperglycemia (high blood sugar):
Hyperglycemia happens when you have too much sugar in your blood. Usually, it means there is not enough insulin to break down the food you eat into energy your body can use. Hyperglycemia can be caused by a fever, an infection, stress, eating more than you should, taking less insulin than prescribed, or it can mean your diabetes is getting worse.
Hyperglycemia can happen with:
- This can happen from:
Insufficient (too little) insulin.
- - injecting too little or no insulin
- - incorrect storage (freezing, excessive heat)
- - use after the expiration date.
- This can happen if you eat larger meals, eat more often, or increase the amount of carbohydrate in your meals. Too much carbohydrate intake.
- Be sure to discuss all your medicines with your healthcare provider. Medicines that affect insulin.Do not start any new medicines until you know how they may affect your insulin dose.
- These medical conditions include fevers, infections, heart attacks, and stress. Medical conditions that affect insulin.
- Injecting insulin the wrong way or in the wrong injection area.
Testing your blood or urine often will let you know if you have hyperglycemia. If your tests are often high, tell your healthcare provider so your dose of insulin can be changed.
Hyperglycemia can be mild or severe. It can progress to diabetic ketoacidosis (DKA) or very high glucose levels (hyperosmolar coma) and result in unconsciousness and death.
Although diabetic ketoacidosis occurs most often in patients with type 1 diabetes,it can also happen in patients with type 2 diabetes who become very sick. Because some patients get few symptoms of hyperglycemia, it is important to check your blood sugar/urine sugar and ketones regularly.
Symptoms of hyperglycemia include:
- confusion or drowsiness
- increased thirst
- decreased appetite, nausea, or vomiting
- rapid heart rate
- increased urination and dehydration (too little fluid in your body).
Symptoms of DKA also include:
- fruity smelling breath
- fast, deep breathing
- stomach area (abdominal) pain.
Severe or continuing hyperglycemia or DKA needs evaluation and treatment right away by your healthcare provider.
Do not use LANTUS to treat diabetic ketoacidosis.
Other possible side effects of LANTUS include:
Serious allergic reactions:
Some times severe, life-threatening allergic reactions can happen with insulin. If you think you are having a severe allergic reaction, get medical help right away. Signs of insulin allergy include:
- rash all over your body
- shortness of breath
- wheezing (trouble breathing)
- fast pulse
- sweating
- low blood pressure.
Reactions at the injection site:
Injecting insulin can cause the following reactions on the skin at the injection site:
- little depression in the skin (lipoatrophy)
- skin thickening (lipohypertrophy)
- red, swelling, itchy skin (injection site reaction).
You can reduce the chance of getting an injection site reaction if you change (rotate) the injection site each time. An injection site reaction should clear up in a few days or a few weeks. If injection site reactions do not go away or keep happening call your healthcare provider.
Swelling of your hands and feet (edema)
Weight gain
Taking certain diabetes pills called thiazolidinediones or "TZDs" with Lantus may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with Lantus. Your healthcare provider should monitor you closely while you are taking TZDs with Lantus. Tell your healthcare provider if you have any new or worse symptoms of heart failure including: Heart Failure.
- shortness of breath
- swelling of your ankles or feet
- sudden weight gain
During treatment with TZDs and Lantus, the TZD dose may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
Tell your healthcare provider if you have any side effects that bother you.
These are not all the side effects of LANTUS. Ask your healthcare provider or pharmacist for more information.
How should I store LANTUS?
-
Unopened SoloStar:
Store new unopened SoloStar disposable insulin pen in a refrigerator (not the freezer) between 36°F to 46°F (2°C to 8°C). Do not freeze LANTUS. Keep LANTUS out of direct heat and light. If a disposable insulin pen has been frozen or overheated, throw it away.
-
Open (In-Use) SoloStar:
Once SoloStar is opened (in-use), SoloStar should be refrigerated but should be kept at room temperature (below 86°F [30°C]) away from direct heat and light. The opened (in-use) SoloStar kept at room temperature must be discarded after 28 days. NOT
These storage conditions are summarized in the following table:
| Not in-use (unopened) Refrigerated
| Not in-use (unopened) Room Temperature
| In-use (opened) Room Temperature (Do not refrigerate)
|
|
|---|---|---|---|
| 3 mL SoloStar disposable insulin device | Until expiration date | 28 days | 28 days |
- Do not use SoloStar with LANTUS after the expiration date stamped on the label.
- Do not use LANTUS if it is cloudy, colored, or if you see particles.
General Information about LANTUS
- Use LANTUS only to treat your diabetes. give or share LANTUS with another person, even if they have diabetes also. It may harm them. Do not
- This leaflet summarizes the most important information about LANTUS. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about LANTUS that is written for healthcare professionals. For more information about LANTUS call 1-800-633-1610 or go to website www.lantus.com.
ADDITIONAL INFORMATION
is a national magazine designed especially for patients with diabetes and their families and is available by subscription from the American Diabetes Association (ADA), P.O. Box 363, Mt. Morris, IL 61054-0363, 1-800-DIABETES (1-800-342-2383). You may also visit the ADA website at www.diabetes.org. DIABETES FORECAST
Another publication, , is available from the Juvenile Diabetes Research Foundation International (JDRF), 120 Wall Street, 19th Floor, New York, New York 10005, 1-800-JDF-CURE (1-800-533-2873). You may also visit the JDRF website at www.jdf.org. COUNTDOWN
To get more information about diabetes, check with your healthcare professional or diabetes educator or visit www.DiabetesWatch.com.
Additional information about LANTUS can be obtained by calling 1-800-633-1610 or by visiting www.lantus.com.
Rev. December 2013
sanofi-aventis U.S. LLC Bridgewater, NJ 08807 A SANOFI COMPANY
©2013 sanofi-aventis U.S. LLC
Lantus and SoloStar are a registered trademark of sanofi-aventis U.S. LLC The brands listed are the trademarks of their respective owners and are not trademarks of sanofi-aventis U.S. LLC
®®
†
LANTUS SOLOSTAR (insulin glargine [rDNA origin] injection)
®®
Instruction Leaflet
Your healthcare professional has decided that SoloStar is right for you. Talk with your healthcare professional about proper injection technique before using SoloStar. ®
Read these instructions carefully before using your SoloStar. If you are not able to follow all the instructions completely on your own, use SoloStar only if you have help from a person who is able to follow the instructions.
Follow these instructions completely each time you use SoloStar to ensure that you get an accurate dose. If you do not follow these instructions you may get too much or too little insulin, which may affect your blood glucose.
SoloStar is a disposable pen for the injection of insulin. Each SoloStar contains in total 300 units of insulin. You can set doses from 1 to 80 units in steps of 1 unit. The pen plunger moves with each dose. The plunger will only move to the end of the cartridge when 300 units of insulin have been given.
Keep this leaflet for future reference.
If you have any questions about SoloStar or about diabetes, ask your healthcare professional, go to www.lantus.com or call sanofi-aventis at 1-800-633-1610.
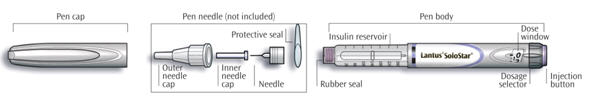
Important information for use of SoloStar:
- Always attach a new needle before each use.
BD Ultra-Fine needles are compatible with SoloStar. These are sold separately and are manufactured by BD. Contact your healthcare professional for further information. ®2
- Always perform the safety test before each injection.
- Do not select a dose or press the injection button without a needle attached.
- This pen is only for your use. Do not share it with anyone else.
- If your injection is given by another person, special caution must be taken by this person to avoid accidental needle injury and transmission of infection.
- Never use SoloStar if it is damaged or if you are not sure that it is working properly.
- Always have a spare SoloStar in case your SoloStar is lost or damaged.
Step 1. Check the insulin
- A.
- Check the label on your SoloStar to make sure you have the correct insulin. The Lantus SoloStar is grey with a purple injection button. ®®
- B.
- Take off the pen cap.
- C.
- Check the appearance of your insulin. Lantus is a clear insulin. Do not use this SoloStar if the insulin is cloudy, colored or has particles.
Step 2. Attach the needle
Always use a new sterile needle for each injection. This helps prevent contamination, and potential needle blocks.
- A.
- Wipe the Rubber Seal with alcohol.
- B.
- Remove the protective seal from a new needle.
- C.
- Line up the needle with the pen, and keep it straight as you attach it (screw or push on, depending on the needle type).
- If the needle is not kept straight while you attach it, it can damage the rubber seal and cause leakage, or break the needle.
Step 3. Perform a Safety test
Performing the safety test ensures that you get an accurate dose by:
perform the safety test before each injection.
Always
- ensuring that pen and needle work properly
- removing air bubbles
- A.
- Select a dose of 2 units by turning the dosage selector.
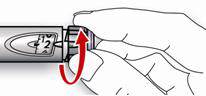
- B.
- Take off the outer needle cap and keep it to remove the used needle after injection. Take off the inner needle cap and discard it.
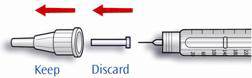
- C.
- Hold the pen with the needle pointing upwards.
- D.
- Tap the insulin reservoir so that any air bubbles rise up towards the needle.
- E.
- Press the injection button all the way in. Check if insulin comes out of the needle tip.
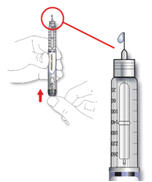
You may have to perform the safety test several times before insulin is seen.
- If no insulin comes out, check for air bubbles and repeat the safety test two more times to remove them.
- If still no insulin comes out, the needle may be blocked. Change the needle and try again.
- If no insulin comes out after changing the needle, your SoloStar may be damaged. Do not use this SoloStar.
Step 4. Select the dose
You can set the dose in steps of 1 unit, from a minimum of 1 unit to a maximum of 80 units. If you need a dose greater than 80 units, you should give it as two or more injections.
- A.
- Check that the dose window shows "0" following the safety test.
- B.
- Select your required dose (in the below, the selected dose is 30 units). If you turn past your dose, you can turn back down.
example
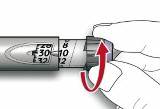
- Do not push the injection button while turning, as insulin will come out.
- You cannot turn the dosage selector past the number of units left in the pen. Do not force the dosage selector to turn. In this case, either you can inject what is remaining in the pen and complete your dose with a new SoloStar or use a new SoloStar for your full dose.
Step 5. Inject the dose
- A.
- Use the injection method as instructed by your healthcare professional.
- B.
- Insert the needle into the skin.
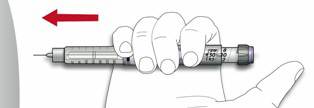
- C.
- Deliver the dose by pressing the injection button in all the way. The number in the dose window will return to "0" as you inject.
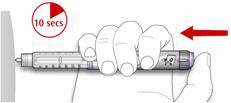
- D.
- Keep the injection button pressed all the way in. This ensures that the full dose will be delivered. Slowly count to 10 before you withdraw the needle from the skin.
Step 6. Remove and discard the needle
Always remove the needle after each injection and store SoloStar without a needle attached. This helps prevent:
- Contamination and/or infection
- Entry of air into the insulin reservoir and leakage of insulin, which can cause inaccurate dosing.
- A.
- Put the outer needle cap back on the needle, and use it to unscrew the needle from the pen. To reduce the risk of accidental needle injury, never replace the inner needle cap.
- If your injection is given by another person, special caution must be taken by this person when removing and disposing the needle. Follow recommended safety measures for removal and disposal of needles (e.g. a one handed capping technique) in order to reduce the risk of accidental needle injury and transmission of infectious diseases.
- B.
- Dispose of the needle safely. Used needles should be placed in sharps containers (such as red biohazard containers), hard plastic containers (such as detergent bottles), or metal containers (such as an empty coffee can). Such containers should be sealed and disposed of properly. If you are giving an injection to a third person, you should remove the needle in an approved manner to avoid needle-stick injuries.
- C.
- Always put the pen cap back on the pen, then store the pen until your next injection.
Storage Instructions
Please check the leaflet for the insulin for complete instructions on how to store SoloStar.
If your SoloStar is in cool storage, take it out 1 to 2 hours before you inject to allow it to warm up. Cold insulin is more painful to inject.
Keep SoloStar out of the reach and sight of children.
Keep your SoloStar in cool storage (36°F - 46°F [2°C – 8°C]) until first use. Do not allow it to freeze. Do not put it next to the freezer compartment of your refrigerator, or next to a freezer pack.
Once you take your SoloStar out of cool storage, for use or as a spare, you can use it for up to 28 days. During this time it can be safely kept at room temperature up to 86°F (30°C). Do not use it after this time.
Do not use SoloStar after the expiration date printed on the label of the pen or on the carton.
Protect SoloStar from light.
Discard your used SoloStar as required by your local authorities.
Maintenance
Protect your SoloStar from dust and dirt.
You can clean the outside of your SoloStar by wiping it with a damp cloth.
Do not soak, wash or lubricate the pen as this may damage it.
Your SoloStar is designed to work accurately and safely. It should be handled with care. Avoid situations where SoloStar might be damaged. If you are concerned that your SoloStar may be damaged, use a new one.
SoloStar in use must not be stored in a refrigerator.
sanofi-aventis U.S. LLC Bridgewater, NJ 08807 A SANOFI COMPANY
November 2013
Date of revision:
©2013 sanofi-aventis U.S. LLC
- 2
- The brands listed are the registered trademarks of their respective owners and are not trademarks of sanofi-aventis U.S. LLC
