MECLIZINE HCL- meclizine hydrochloride tablets tablet
Trigen Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
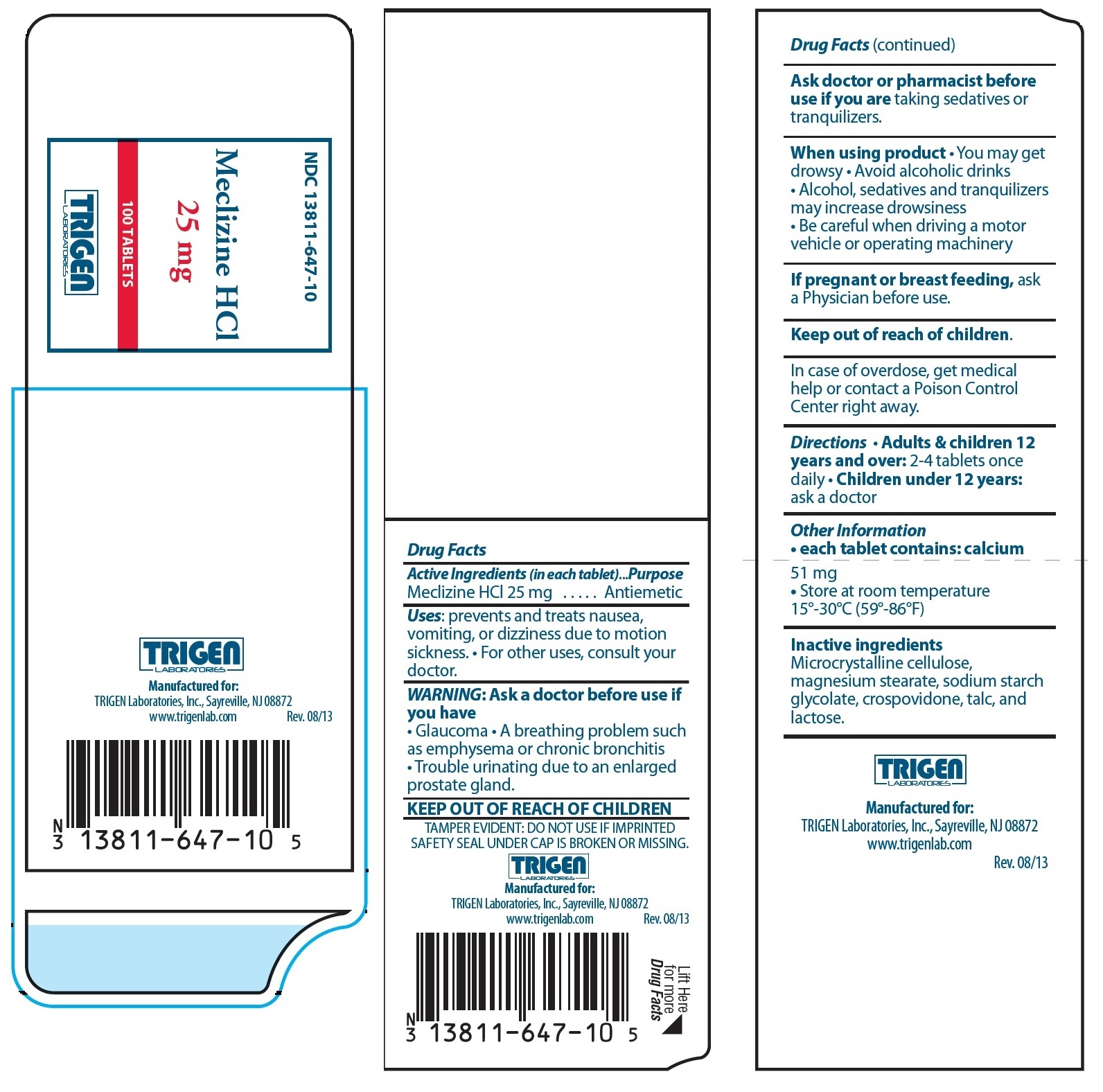
Active Ingredients (in each tablet)
Meclizine HCl 25 mg
Uses:
prevents and treats nausea, vomiting, or dizziness due to motion sickness.• For other uses, consult your doctor.
WARNING: Ask a doctor before use if you have
• Glaucoma • A breathing problem such as emphysema or chronic bronchitis • Trouble urinating due to an enlarged prostate gland.
KEEP OUT OF REACH OF CHILDREN
Ask doctor or pharmacist before use if you are taking sedatives or tranquilizers.
When using product
• You may get drowsy • Avoid alcoholic drinks • Alcohol, sedatives and tranquilizers may increase drowsiness • Be careful when driving a motor vehicle or operating machinery
If pregnant or breast feeding, ask a Physician before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
• Adults & children 12 years and over: 2-4 tablet once daily
• Children under 12 years: ask a doctor
Other Information
• each tablet contains: calcium 51 mg
• Store at room temperature 15°-30°C (59°-86°F)
Inactive ingredients
Microcrystalline cellulose, magnesium stearate, sodium starch glycolate, crospovidone, talc, and lactose.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
TRIGEN
LABORATORIES
Manufactured for:
TRIGEN Laboratories, Inc., Sayreville, NJ 08872
www.trigenlab.com
Rev. 08/13
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Label
NDC 13811-647-10
Meclizine HCl
25 mg
100 TABLETS
TRIGEN
LABORATORIES
Manufactured for:
TRIGEN Laboratories, Inc., Sayreville, NJ 08872
www.trigenlab.com
Rev. 08/13
Trigen Laboratories, Inc.