CAUTION: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or
on the order of a licensed veterinary.
For Use in Swine and Cattle Feeds Only
|
Do Not Feed Undiluted |
Active Drug Ingredient: Tilmicosin (as tilmicosin phosphate) 90.7 g per lb (200 g per kg).
Inert Ingredients: Corncobs, macrogolglycerol ricinoleate.
Description: Tilmovet® (tilmicosin) 90 is a formulation of the antibiotic tilmicosin.
Tilmicosin is produced semi-synthetically and is in the macrolide class of antibiotics.
Each kilogram of Type A Medicated Article contains 200 grams (0.44 lbs) of tilmicosin
adsorbed onto ground corncobs.
Indications:
Swine: For the control of swine respiratory disease (SRD) associated with Actinobacillus
pleuropneumoniae and Pasteurella multocida.
Cattle: For the control of bovine respiratory disease (BRD) associated with Mannheimia
haemolytica, Pasteurella multocida and Histophilus somni in groups of beef and nonlactating
dairy cattle, where active BRD has been diagnosed in at least 10% of the animals
in the group.
Feeding Directions:
Swine: Tilmicosin is to be fed continuously at 181 grams to 363 grams per ton (200 ppm to
400 ppm) of Type C medicated feed as the sole ration for a 21-day period, beginning
approximately 7 days before an anticipated disease outbreak.
Cattle: Tilmicosin is to be fed continuously for a single, 14 day period at 568 grams to 757
grams (626 ppm to 834 ppm) per ton on a 100% dry matter basis of Type C medicated feed
as the sole ration to provide 12.5 mg/kg of body weight/day.
IMPORTANT: Must be thoroughly mixed in swine or cattle feeds before use.
Mixing Directions:
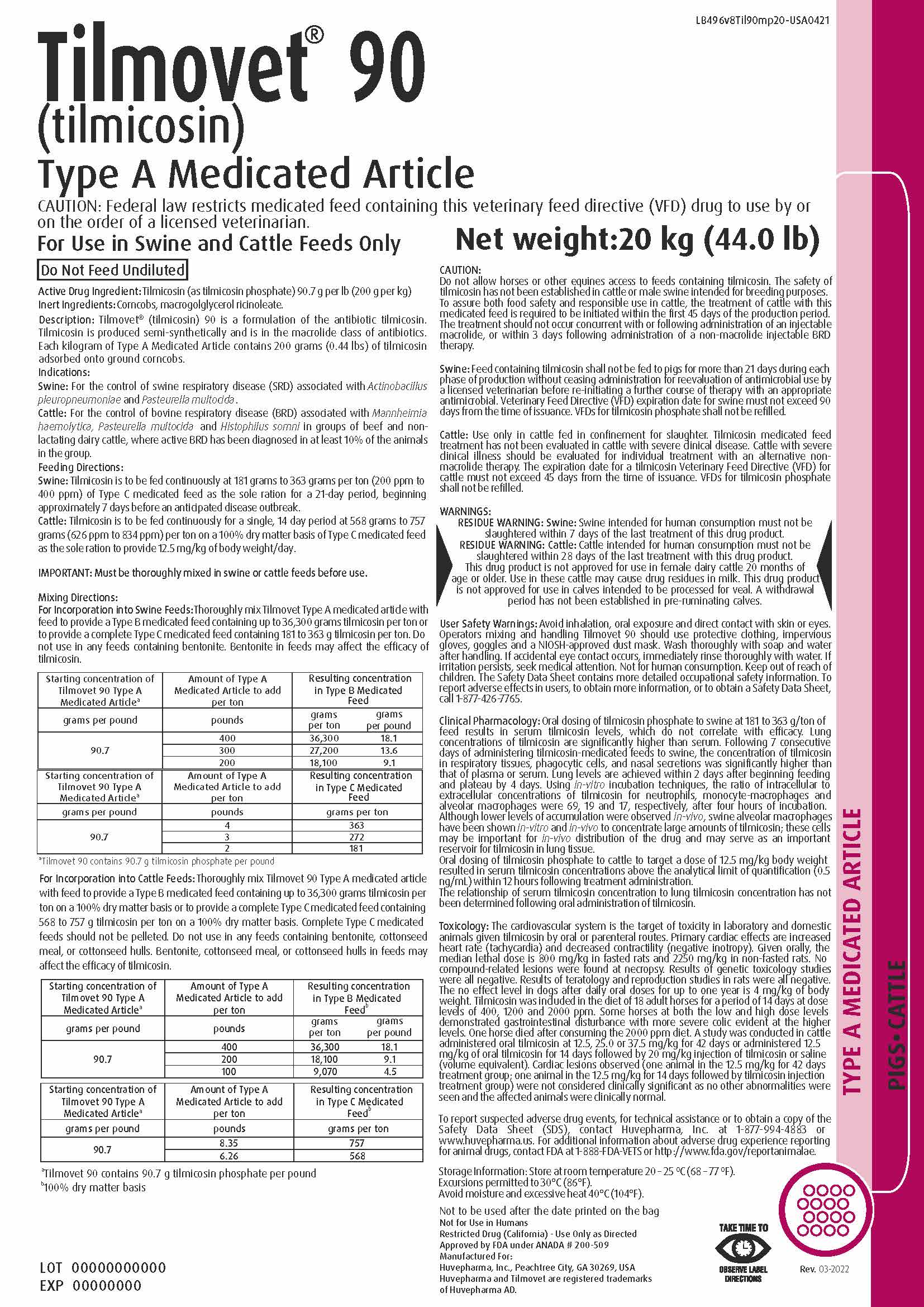
For Incorporation into Swine Feeds: Thoroughly mix Tilmovet Type A medicated article with
feed to provide a Type B medicated feed containing up to 36,300 grams tilmicosin per ton or
to provide a complete Type C medicated feed containing 181 to 363 g tilmicosin per ton. Do
not use in any feeds containing bentonite. Bentonite in feeds may affect the efficacy of
tilmicosin.
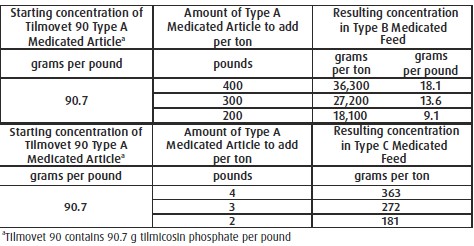
For Incorporation into Cattle Feeds: Thoroughly mix Tilmovet 90 Type A medicated article
with feed to provide a Type B medicated feed containing up to 36,300 grams tilmicosin per
ton on a 100% dry matter basis or to provide a complete Type C medicated feed containing
568 to 757 g tilmicosin per ton on a 100% dry matter basis. Complete Type C medicated
feeds should not be pelleted. Do not use in any feeds containing bentonite, cottonseed
meal, or cottonseed hulls. Bentonite, cottonseed meal, or cottonseed hulls in feeds may
affect the efficacy of tilmicosin.
LOT
EXP
Net weight:20 kg (44.0 lb)
CAUTION:
Do not allow horses or other equines access to feeds containing tilmicosin. The safety of
tilmicosin has not been established in cattle or male swine intended for breeding purposes.
To assure both food safety and responsible use in cattle, the treatment of cattle with this
medicated feed is required to be initiated within the first 45 days of the production period.
The treatment should not occur concurrent with or following administration of an injectable
macrolide, or within 3 days following administration of a non-macrolide injectable BRD
therapy.
Swine: Feed containing tilmicosin shall not be fed to pigs for more than 21 days during each
phase of production without ceasing administration for reevaluation of antimicrobial use by
a licensed veterinarian before re-initiating a further course of therapy with an appropriate
antimicrobial. Veterinary Feed Directive (VFD) expiration date for swine must not exceed 90
days from the time of issuance. VFDs for tilmicosin phosphate shall not be refilled.
Cattle: Use only in cattle fed in confinement for slaughter. Tilmicosin medicated feed
treatment has not been evaluated in cattle with severe clinical disease. Cattle with severe
clinical illness should be evaluated for individual treatment with an alternative nonmacrolide
therapy. The expiration date for a tilmicosin Veterinary Feed Directive (VFD) for
cattle must not exceed 45 days from the time of issuance. VFDs for tilmicosin phosphate
shall not be refilled.
WARNINGS:
RESIDUE WARNING: Swine: Swine intended for human consumption must not be
slaughtered within 7 days of the last treatment of this drug product.
RESIDUE WARNING: Cattle: Cattle intended for human consumption must not be
slaughtered within 28 days of the last treatment with this drug product.
This drug product is not approved for use in female dairy cattle 20 months of
age or older. Use in these cattle may cause drug residues in milk. This drug product
is not approved for use in calves intended to be processed for veal. A withdrawal
period has not been established in pre-ruminating calves.
User Safety Warnings: Avoid inhalation, oral exposure and direct contact with skin or eyes.
Operators mixing and handling Tilmovet 90 should use protective clothing, impervious
gloves, goggles and a NIOSH-approved dust mask. Wash thoroughly with soap and water
after handling. If accidental eye contact occurs, immediately rinse thoroughly with water. If
irritation persists, seek medical attention. Not for human consumption. Keep out of reach of
children. The Safety Data Sheet contains more detailed occupational safety information. To
report adverse effects in users, to obtain more information, or to obtain a Safety Data Sheet,
call 1-877-426-7765.
Clinical Pharmacology: Oral dosing of tilmicosin phosphate to swine at 181 to 363 g/ton of
feed results in serum tilmicosin levels, which do not correlate with efficacy. Lung
concentrations of tilmicosin are significantly higher than serum. Following 7 consecutive
days of administering tilmicosin-medicated feeds to swine, the concentration of tilmicosin
in respiratory tissues, phagocytic cells, and nasal secretions was significantly higher than
that of plasma or serum. Lung levels are achieved within 2 days after beginning feeding
and plateau by 4 days. Using in-vitro incubation techniques, the ratio of intracellular to
extracellular concentrations of tilmicosin for neutrophils, monocyte-macrophages and
alveolar macrophages were 69, 19 and 17, respectively, after four hours of incubation.
Although lower levels of accumulation were observed in-vivo, swine alveolar macrophages
have been shown in-vitro and in-vivo to concentrate large amounts of tilmicosin; these cells
may be important for in-vivo distribution of the drug and may serve as an important
reservoir for tilmicosin in lung tissue.
Oral dosing of tilmicosin phosphate to cattle to target a dose of 12.5 mg/kg body weight
resulted in serum tilmicosin concentrations above the analytical limit of quantification (0.5
ng/mL) within 12 hours following treatment administration.
The relationship of serum tilmicosin concentration to lung tilmicosin concentration has not
been determined following oral administration of tilmicosin.
Toxicology: The cardiovascular system is the target of toxicity in laboratory and domestic
animals given tilmicosin by oral or parenteral routes. Primary cardiac effects are increased
heart rate (tachycardia) and decreased contractility (negative inotropy). Given orally, the
median lethal dose is 800 mg/kg in fasted rats and 2250 mg/kg in non-fasted rats. No
compound-related lesions were found at necropsy. Results of genetic toxicology studies
were all negative. Results of teratology and reproduction studies in rats were all negative.
The no effect level in dogs after daily oral doses for up to one year is 4 mg/kg of body
weight. Tilmicosin was included in the diet of 18 adult horses for a period of 14 days at dose
levels of 400, 1200 and 2000 ppm. Some horses at both the low and high dose levels
demonstrated gastrointestinal disturbance with more severe colic evident at the higher
levels. One horse died after consuming the 2000 ppm diet. A study was conducted in cattle
administered oral tilmicosin at 12.5, 25.0 or 37.5 mg/kg for 42 days or administered 12.5
mg/kg of oral tilmicosin for 14 days followed by 20 mg/kg injection of tilmicosin or saline
(volume equivalent). Cardiac lesions observed (one animal in the 12.5 mg/kg for 42 days
treatment group; one animal in the 12.5 mg/kg for 14 days followed by tilmicosin injection
treatment group) were not considered clinically significant as no other abnormalities were
seen and the affected animals were clinically normal.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the
Safety Data Sheet (SDS), contact Huvepharma, Inc. at 1-877-994-4883 or
www.huvepharma.us. For additional information about adverse drug experience reporting
for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
Storage Information: Store at room temperature 20 – 25 ºC (68 – 77 ºF).
Excursions permitted to 30°C (86°F).
Avoid moisture and excessive heat 40°C (104°F).
Not to be used after the date printed on the bag
Not for Use in Humans
Restricted Drug (California) - Use Only as Directed
Approved by FDA under ANADA # 200-509
Manufactured For:
Huvepharma, Inc., Peachtree City, GA 30269, USA
Huvepharma and Tilmovet are registered trademarks
of Huvepharma AD.

Rev. 03-2022