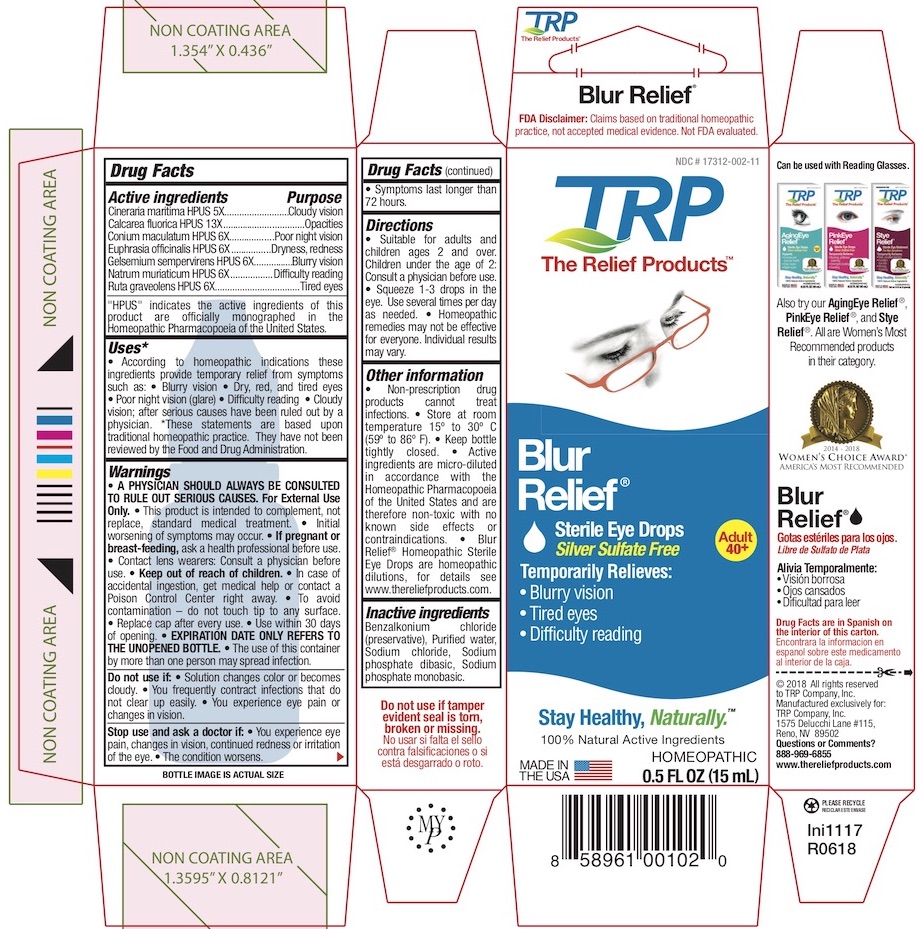
| Active Ingredients | Purpose |
| Cineraria Maritima HPUS 5X | Cloudy Vision |
| Calcarea fluorica HPUS 13X | Opacities |
| Conium Maculatum HPUS 6x | Poor Night Vision |
| Euphrasia (Eyebright) HPUS 6x | Dryness, Redness |
| Gelsemium HPUS 6x | Blurry vision |
| Natrum Muriaticum HPUS 6x | Difficulty reading |
| Ruta Graveolens HPUS 6x | Tired Eyes |
The letters HPUS indicate that the components of this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Uses:*
According to homeopathic indications these ingredients provide temporary relief from symptoms such as:
• Blurry Vision
• Dry, Red & Tired Eyes
• Poor Night Vision (Glare)
• Difficulty reading after serious causes have been ruled out by a physician.
*These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
Warnings:
A PHYSICIAN SHOULD ALWAYS BE CONSULTED TO RULE OUT SERIOUS CAUSES.
For External Use Only.
- This product is intended to complement not replace, standard medical treatment.
- Initial worsening of symptoms may occur.
- Physician should always be consulted to rule out serious conditions.
- Contact lens wearers consult physician prior to using.
- To avoid contamination - do not touch tip to any surface.
- Replace cap after every use.
- Use within 30 days of opening.
- EXPIRATION DATE ONLY REFERS TO THE UNOPENED BOTTLE.
- The use of this container by more than one person may spread infection.
Do not use:
• If solution changes color or becomes cloudy. • If you frequently contract infections that do not clear up easily. • If you experience eye pain or changes in vision.
Stop use and ask a doctor if:
• You experience eye pain, changes in vision, continued redness or irritation of the eye. • The condition worsens. • The condition persists for more than 72 hours.
Directions:
• Suitable for adults and children ages 2 and over. • Children under the age of 2: Consult a physician before use. • Squeeze 1-3 drops in the eye. • Use several times per day as needed. • Use within 30 days of opening. • Expiration date only refers to unopened bottle. • Homeopathic remedies may not be effective for everyone. • May take up to 60 days to see results.
Other information:
- There are no known contraindications
- Active ingredients are micro-diluted in accordance with the Homeopathic Pharmacopoeia of the United States and are therefore non-toxic with no known side effects. Blur Relief Homeopathic Sterile Eye Drops are homeopathic dilutions: see www.thereliefproducts.com for details.
- Store at room temperature 15° to 30° C (59° to 86° F).
- Keep bottle tightly closed