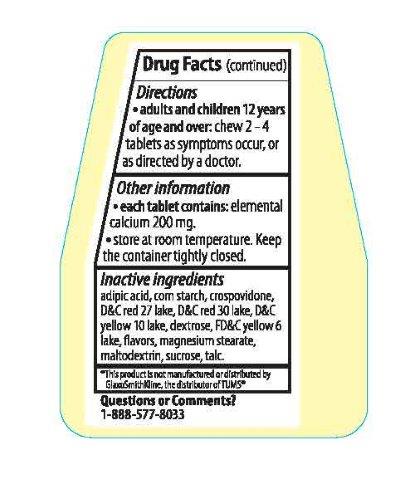
adipic acid, corn starch, crospovidone, D&C red 27 lake, D&C red 30 lake, D&C yellow 10 lake, dextrose, FD&C yellow 6 lake, flavors, magnesium stearate, maltodextrin, sucrose, talc.
Adults and children 12 years of age and over: chew 2-4 tablets as symptoms occur, or as directed by a doctor.
Ask a doctor or Pharmacist before use if you are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product do not take more than 15 tablets in a 24-hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a doctor.
If pregnant or breast-feeding,ask a health professional before use.
Keep out of reach of children.