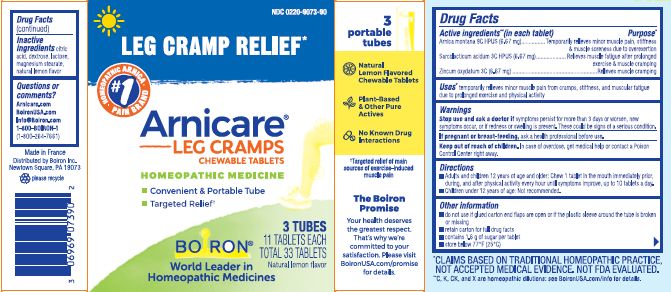
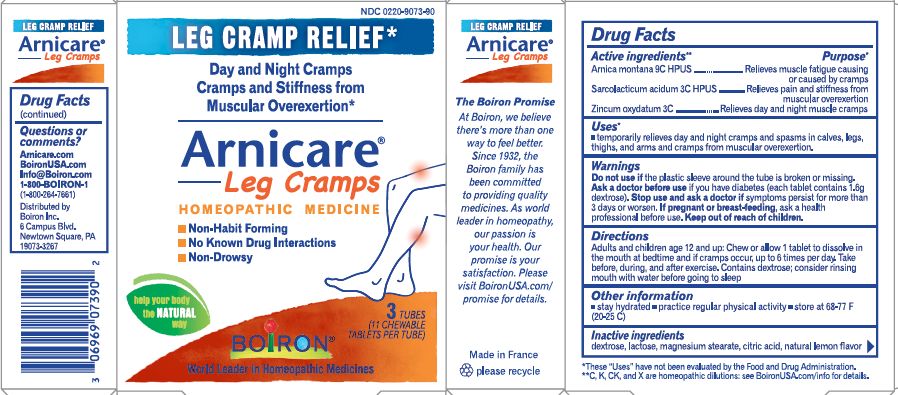
Active Ingredients**
Arnica montana 9C HPUS (6.67 mg)
Sarcolacticum acidum 3C HPUS (6.67 mg)
Zincum oxydatum 3C (6.67 mg)
Purpose*
Arnica montana 9C HPUS ... Temporarily relieves minor pain, stiffness & muscle soreness due to overexertion
Sarcolacticum acidum 3C HPUS ... Relieves muscle fatigue after prolonged exercise & muscle cramping
Zincum oxydatum 3C ... Relieves muscle cramping
Uses*
temporarily relieves minor muscle pain from cramps, stiffness, and muscular fatigue due to prolonged exercise and physical activity
Stop use and ask a doctor if symptoms persist for more than 3 days or worsen, new symptoms occur, or if redness or swelling is present. These could be signs of a serious condition.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age and older: Chew 1 tablet in the mouth immediately prior, during, and after physical activity every hour until symptoms improve, up to 10 tablets a day.
Children under 12 years of age: Not recommended.
do not use if glued carton end flaps are open or if the plastic sleeve around the tube is broken or missing
retain carton for full drug facts
contains 1.6 g of sugar per tablet
store below 77° F (25°C)
3 Tubes 11 Tablets Each Total 33 Tablets
Natural lemon flavor
No Known Drug Interactions
Leg Cramp Relief*
Cramps Spasms*
Target Relief of main sources of exercise-induced muscle pain
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
*C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.