DESCRIPTION
Maropitant is a neurokinin (NK1) receptor antagonist that blocks the pharmacological action of substance P in the central nervous system (CNS). Maropitant is the non-proprietary designation for a substituted quinuclidine. The empirical formula is C32H40N2O C6H8O7 H2O and the molecular weight 678.81. The chemical name is (2S,3S)-2-benzhydryl-N-(5-tert-butyl-2- methoxybenzyl) quinuclidin-3-amine citrate monohydrate. Each mL of CERENIA Injectable Solution contains 10 mg maropitant, 63 mg sulphobutylether-beta-cyclodextrin, 3.3 mg meta-cresol and water for injection.
The chemical structure of maropitant citrate is:
INDICATIONS
Dogs: CERENIA (maropitant citrate) Injectable Solution is indicated for the prevention and treatment of acute vomiting in dogs.
Cats: CERENIA (maropitant citrate) Injectable Solution is indicated for the treatment of vomiting in cats.
DOSAGE AND ADMINISTRATION
Use of refrigerated product may reduce the pain response associated with subcutaneous injection.
Dogs
For Prevention and Treatment of Acute Vomiting in Dogs:
Dogs 2-4 Months of Age: Administer CERENIA Injectable Solution subcutaneously at 1 mg/kg (0.45 mg/lb) equal to 0.1 mL/kg (0.1 mL/2.2 lb) of body weight once daily for up to 5 consecutive days.
Dogs 4 months of Age and Older: Administer CERENIA Injectable Solution intravenously over 1-2 minutes or subcutaneously at 1 mg/kg (0.45 mg/lb) equal to 0.1 mL/1 kg (1 mL/22 lb) of body weight once daily for up to 5 consecutive days.
In dogs that are actively vomiting, it is recommended to initiate treatment with CERENIA Injectable Solution. Thereafter, CERENIA Tablets may be used for the prevention of acute vomiting at 2 mg/kg once daily. (See CERENIA Tablets package insert for complete prescribing information).
For Prevention of Vomiting in Dogs 4 months of Age and Older Caused by Emetogenic Medications or Chemotherapeutic Agents: Administer CERENIA Injectable Solution intravenously over 1-2 minutes or subcutaneously at 1 mg/kg (0.45 mg/lb) of body weight one time, 45-60 minutes prior to use of emetogenic medications or chemotherapeutic agents
Cats
For Treatment of Vomiting in Cats 4 Months of Age and Older: Administer CERENIA Injectable Solution intravenously over 1-2 minutes or subcutaneously at 1 mg/kg (0.45 mg/lb) equal to 0.1 mL/kg (0.1 mL/2.2 lb) of body weight once daily for up to 5 consecutive days.
The underlying cause of acute vomiting should be identified and addressed in dogs and cats that receive CERENIA Injectable Solution. If vomiting persists despite treatment, the case should be re-evaluated.
WARNINGS
Not for use in humans. Keep out of reach of children. In case of accidental injection or exposure, seek medical advice. Topical exposure may elicit localized allergic skin reactions in some individuals. Repeated or prolonged exposure may lead to skin sensitization. In case of accidental skin exposure, wash with soap and water. CERENIA is also an ocular irritant. In case of accidental eye exposure, flush with water for 15 minutes and seek medical attention.
In puppies younger than 11 weeks of age, histological evidence of bone marrow hypocellularity was observed at higher frequency and greater severity in puppies treated with CERENIA compared to control puppies. In puppies 16 weeks and older, bone marrow hypocellularity was not observed (see ANIMAL SAFETY).
PRECAUTIONS
The safe use of CERENIA Injectable Solution has not been evaluated in dogs or cats with gastrointestinal obstruction or that have ingested toxins.
Use with caution in patients with hepatic dysfunction because CERENIA Injectable Solution is metabolized by CYP3A, CYP2D15(dogs) and CYP1A (cats) enzymes (see Pharmacokinetics). The influence of concomitant drugs that may inhibit the metabolism of CERENIA Injectable Solution has not been evaluated. CERENIA Injectable Solution is highly protein bound. Use with caution with other medications that are highly protein bound. The concomitant use of CERENIA Injectable Solution with other protein bound drugs has not been studied in dogs or cats. Commonly used protein bound drugs include NSAIDs, cardiac, anticonvulsant, and behavioral medications. Drug compatibility should be monitored in patients requiring adjunctive therapy.
The safe use of CERENIA Injectable Solution has not been evaluated in dogs or cats used for breeding, or in pregnant or lactating bitches or queens.
ADVERSE REACTIONS
DOGS
In a US field study for the prevention and treatment of vomiting associated with administration of cisplatin for cancer chemotherapy, the following adverse reactions were reported in 77 dogs treated with CERENIA Injectable Solution at 1 mg/kg subcutaneously or 41 dogs treated with placebo:
| Adverse Reaction | Placebo (n=41) | CERENIA (n=77) | ||
|---|---|---|---|---|
| # dogs | % occur | # dogs | % occur | |
| Diarrhea | 1 | 2.4 | 6 | 7.8 |
| Anorexia | 0 | 0 | 4 | 5.2 |
| Injection site reaction (swelling, pain upon injection) | 0 | 0 | 3 | 4 |
| Lethargy | 1 | 2.4 | 2 | 2.6 |
The following adverse reactions were reported during the course of a US field study for the prevention and treatment of acute vomiting in dogs treated with 1 mg/kg CERENIA Injectable Solution subcutaneously and/or CERENIA Tablets at a minimum of 2 mg/kg orally once daily for up to 5 consecutive days:
| Adverse Reaction | Placebo (n=69) | CERENIA (n=206) | ||
|---|---|---|---|---|
| # dogs | % occur | # dogs | % occur | |
| Death during study | 4 | 5.8 | 10 | 4.9 |
| Euthanized during study | 0 | 0 | 2 | 1 |
| Diarrhea | 6 | 8.7 | 8 | 3.9 |
| Hematochezia/bloody stool | 5 | 7.2 | 4 | 1.9 |
| Anorexia | 2 | 2.9 | 3 | 1.5 |
| Otitis/Otorrhea | 0 | 0 | 3 | 1.5 |
| Endotoxic Shock | 1 | 1.4 | 2 | 1 |
| Hematuria | 0 | 0 | 2 | 1 |
| Excoriation | 0 | 0 | 2 | 1 |
Other clinical signs were reported but were <0.5% of dogs.
Adverse reactions seen in a European field study included ataxia, lethargy and injection site soreness in one dog treated with CERENIA Injectable Solution.
Post-Approval Experience (Rev. 2015)
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse events are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported for dogs are listed in decreasing order of reporting frequency for CERENIA Injectable Solution: Pain/vocalization upon injection, depression/lethargy, anorexia, anaphylaxis/anaphylactoid reactions (including swelling ofthe head/face), ataxia, convulsions, hypersalivation, tremors, fever, dyspnea, collapse/loss of consciousness, recumbency, injection site reactions (swelling, inflammation) and sedation.
Cases of death (including euthanasia) have been reported.
CATS
The following adverse reactions were reported during the course of a US field study for the treatment of vomiting in cats treated with 1 mg/kg CERENIA Injectable Solution subcutaneously once daily for up to five consecutive days:
| Adverse Reaction | Placebo (n=62) | CERENIA (n=133) | ||
|---|---|---|---|---|
| # cats | % occur | # cats | % occur | |
| Moderate Response to Injection*,† | 1 | 1.6 | 30 | 22.6 |
| Significant Response to Injection*,‡ | 1 | 1.6 | 15 | 11.3 |
| Fever/Pyrexia | 2 | 3.2 | 2 | 1.5 |
| Dehydration | 0 | 0 | 3 | 2.3 |
| Lethargy | 0 | 0 | 2 | 1.5 |
| Anorexia | 0 | 0 | 1 | 0.8 |
| Hematuria | 0 | 0 | 1 | 0.8 |
| Hypersalivation | 0 | 0 | 1 | 0.8 |
| Injection site swelling | 1 | 1.6 | 0 | 0 |
1The clinician observed and graded each cat’s response to injection.
2 Cat objected to the injection by retreating and vocalizing
3 Cat objected to the injection by retreating, hissing, scratching, and vocalization
Post-Approval Experience (Rev. 2015)
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse events are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported for cats are listed in decreasing order of reporting frequency for CERENIA Injectable Solution: Depression/lethargy, anorexia, hypersalivation, pain/vocalization upon injection, dyspnea, ataxia, fever, recumbency, vomiting, panting, convulsion, and muscle tremor.
Cases of death (including euthanasia) have been reported.
To report suspected adverse events, for technical assistance or to obtain a copy of the SDS, contact Zoetis Inc. at 1-888-963-8471 or www.zoetis.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
CLINICAL PHARMACOLOGY:
Pharmacodynamics:
Vomiting is a complex process coordinated centrally by the emetic center which consists of several brainstem nuclei (area postrema, nucleus tractus solitarius, dorsal motor nucleus of the vagus) that receive and integrate sensory stimuli from central and peripheral sources and chemical stimuli from the circulation and the cerebro-spinal fluid. Maropitant is a neurokinin 1 (NK1) receptor antagonist which acts by inhibiting the binding of substance P, a neuropeptide of the tachykinin family. Substance P is found in significant concentrations in the nuclei comprising the emetic center and is considered the key neurotransmitter involved in emesis.1 By inhibiting the binding of substance P within the emetic center, maropitant provides broad-spectrum effectiveness against neural (central) and humoral (peripheral) causes of vomiting. In vivo model studies in dogs have shown that maropitant has antiemetic effectiveness against both central and peripheral emetogens including apomorphine, cisplatin, and syrup of ipecac.
Pharmacokinetics:
CERENIA Injectable Solution is formulated using sulphobutyletherß-cyclodextrin (SBECD), which exhibits enhanced binding to maropitant at refrigerated temperatures. The enhanced binding affinity reverses rapidly upon warming.
DOGS
The pharmacokinetic (PK) characterization associated with maropitant after a single oral (PO), intravenous (IV), or subcutaneous (SC) dose administration in adult Beagle dogs is provided in the table below.
| PKParameter | SC at 1 mg/kg (n=8) | IV at 1 mg/kg (n=8) | PO at 2 mg/kg (n=8) | PO at 8 mg/kg (n=8) |
|---|---|---|---|---|
| AUC0-inf (hr*ng/mL) | 759.08±189.49 | 693.83±137.25 | 561±322 | 7840±5600 |
| Cmax (ng/mL) | 102.99±46.06 | 296.62±60.77 | 81±32 | 776±604 |
| T1/2 (hr) |
8.84a (6.15-20.48) |
6.85a (4.87 -11.30) | 4.03 (2.48–7.09) | 5.46 (3.39–7.65) |
| Tmax (hr) |
0.56±0.40 | n/a | 1.9±0.5 | 1.7±0.7 |
a Harmonic mean
The absolute bioavailability of maropitant was much higher following SC injection (91% at 1 mg/kg) than after PO administration (24% at 2 mg/kg). Oral bioavailability may be underestimated due to the presence of nonlinear kinetics and the resulting longer T1/2 seen after intravenous (IV) administration. Although hepatic firstpass metabolism contributed to the relatively low bioavailability after an oral dose, prandial status does not significantly affect the extent of oral bioavailability. Greater than dose-proportional drug exposure can be expected with an increase in dose (1–16 mg/kg PO). Systemic clearance of maropitant following IV administration was 1499.13 mL/hr/kg at a dose of 1 mg/kg. An accumulation ratio of 1.5 was observed following once-daily use of maropitant for five consecutive days at 1 (SC) or 2 mg/kg (PO). Urinary recovery of maropitant and its major metabolitewas minimal (<1% each). The hepatic metabolism of maropitant involves two cytochrome P-450 isoenzymes: CYP2D15 and CYP3A12. Based on in vitro enzyme kinetics data, it is believed that the non-linear kinetics may be partially associated with saturation of the low capacity enzyme (CYP2D15). However as doses increase (20–50 mg/kg PO), dose proportionality is re-established.
Based upon in vitro enzyme kinetics, involvement of a high capacity enzyme (CYP3A12) may contribute to this return to dose linearity. Plasma protein binding of maropitant was high (99.5%).
Based on differences in plasma trough concentrations from a single study, the exposure of 10 week old puppies to maropitant may be lower than that observed in adult dogs, particularly after doses of 1 or 2 mg/kg.
CATS
The pharmacokinetic characterization associated with maropitant after a single subcutaneous (SC) or intravenous (IV) dose administration in cats is provided in the table below.
Pharmacokinetic Parameters for a Single Dose in 6-7 Month Old Cats
(Mean±SD or Mean and Range)
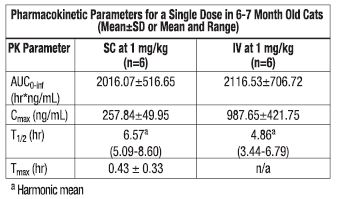
There appears to be an age-related effect on the pharmacokinetics of maropitant in cats; kittens (4 months) have a higher clearance than adults. In multiple IV and SC studies, the mean maropitant half-life in kittens (4-7 months old) is 7.83 hours, compared to 17.2 hours in adults. The mean bioavailability of maropitant after subcutaneous administration in cats was 91.3%. The mean total body clearance (CL) and volume of distribution at steady-state (Vss) determined after IV administration of 1.0 mg/kg to 6 cats was 510 (388 to 603) mL/hr/kg and 2.3 (1.4 to 3.6) L/kg, respectively. Maropitant displays linear kinetics when administered SC within the 0.25–3 mg/kg dose range. Following SC administration of once daily doses of 1 mg/kg body weight for 5 consecutive days, accumulation was 250%. Maropitant undergoes cytochrome P450 (CYP) metabolism in the liver. CYP1A and CYP3A-related enzymes were identified as the feline isoforms involved in the hepatic biotransformation of maropitant. Renal and fecal clearances are minor routes of elimination for maropitant, with less than 1% of a 1 mg/kg SC dose appearing in the urine or feces as maropitant. For the major metabolite, 10.4% of the maropitant dose was recovered in urine and 9.3% in feces. Plasma protein binding of maropitant in cats was estimated to be 99.1%.
EFFECTIVENESS
DOGS
In laboratory model studies, CERENIA Injectable Solution administered at 1 mg/kg in Beagle dogs reduced the number of emetic events associated with established neural (central) and humoral (peripheral) stimuli. Following administration of apomorphine (central emetic stimuli), vomiting was observed in 16.7% (2 of 12) of dogs treated with CERENIA Injectable Solution and 83.3% (10 of 12) of placebo-treated dogs. Following administration of syrup of ipecac (peripheral emetic stimuli) vomiting was observed in 25% (3 of 12) of dogs treated with CERENIA Injectable Solution and in 100% (12 of 12) of dogs treated with placebo.
In a study of veterinary cancer patients, dogs were treated with CERENIA Injectable Solution or placebo either 1 hour prior to cisplatin (prevention) or after the first vomiting episode following cisplatin (treatment) and monitored for 5 hours. In the groups evaluated for prevention of vomiting, 94.9% (37/39) of the dogs administered CERENIA Injectable Solution and 4.9% (2/41) of the dogs administered placebo did not vomit. In the groups evaluated for treatment, 21% (8/38) of the dogs administered CERENIA Injectable Solution and 5.1% (2/39) of the dogs administered placebo had no further episodes of vomiting following treatment.
| Number of Vomiting Episodes | Dogs with Vomiting Episodes* (% of Dogs) | |||
|---|---|---|---|---|
| Treatment of Vomiting | Prevention of Vomiting | |||
| Placebo (n=39†) | CERENIA (n=38†) | Placebo (n=41) | CERENIA (n=39) |
|
|
||||
| 0 | 2 (5.1) | 8 (21.1) | 2 (4.9) | 37 (94.9) |
| 1 | 3 (7.7) | 7 (18.4) | 2 (4.9) | 1 (2.6) |
| 2 | 4 (10.3) | 6 (15.8) | 3 (7.3) | 1 (2.6) |
| 3 | 3 (7.7) | 6 (15.8) | 4 (9.8) | 0 (0) |
| 4 | 4 (10.3) | 4 (10.5) | 3 (7.3) | 0 (0) |
| 5 | 2 (5.1) | 5 (13.2) | 4 (9.8) | 0 (0) |
| 6 | 14 (35.9) | 1 (2.6) | 1 (2.4) | 0 (0) |
| 7 | 2 (5.1) | 1 (2.6) | 12 (29.3) | 0 (0) |
| 8 | 2 (5.1) | 0 (0) | 5 (12.2) | 0 (0) |
| 9 | 2 (5.1) | 0 (0) | 2 (4.9) | 0 (0) |
| 10 | 0 (0) | 0 (0) | 2 (4.9) | 0 (0) |
| 11 | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) |
| 12 | NA | NA | 1 (2.4) | 0 (0) |
* Dogs that exhibited an unacceptable level of vomiting (6 events) were withdrawn from the study and treated with another antiemetic.
** There were initially 41 and 42 dogs treated with either placebo or CERENIA Injectable Solution, respectively. However, if a dog did not vomit following cisplatin therapy, it did not receive a post-cisplatin treatment with either placebo or CERENIA Injectable Solution, and hence it was not considered in the therapeutic evaluation.
In a study of 275 canine patients presented to veterinary hospitals with a history of acute vomiting, dogs were initially administered CERENIA Injectable Solution or placebo on Day 0. Following the initial dose, dogs allocated to the CERENIA group were treated with either CERENIA Tablets at a minimum of 2 mg/kg orally or Injectable Solution at 1 mg/kg subcutaneously once daily at the discretion of the clinician. Dogs allocated to the placebo group were treated using either an injectable placebo solution or placebo tablets once daily at the discretion of the clinician. Of the 199 dogs included in the analysis for effectiveness, 27 of 54 dogs (50%) in the placebo group displayed vomiting at some time during the study and 31 of 145 dogs (21.4%) in the CERENIA-treated group displayed vomiting during the study period.
| Days | Treatment | Route | # dogs | # vomited | % vomited |
|---|---|---|---|---|---|
|
|||||
| Day 0 | Placebo (54) | SC | 54 | 15 | 28% |
| CERENIA (145) | SC | 145 (143*) | 14 | 10% | |
| Day 1 | Placebo (45) | PO | 22 | 3 | 14% |
| SC | 23 | 16 | 70% | ||
| CERENIA (108) | PO | 67 | 2 | 3% | |
| SC | 41 | 16 | 39% | ||
| Day 2 | Placebo (16) | PO | 7 | 2 | 29% |
| SC | 9 | 6 | 67% | ||
| CERENIA (37) | PO | 24 | 0 | 0% | |
| SC | 13 | 8 | 62% | ||
| Day 3 | Placebo (6) | PO | 2 | 0 | 0% |
| SC | 4 | 1 | 25% | ||
| CERENIA (21) | PO | 14 | 0 | 0% | |
| SC | 7 | 5 | 71% | ||
| Day 4 | Placebo (2) | PO | 1 | 0 | 0% |
| SC | 1 | 1 | 100% | ||
| CERENIA (7) | PO | 5 | 0 | 0% | |
| SC | 2 | 1 | 50% | ||
| Day 5 | CERENIA (1) | SC | 1 | 0 | 0% |
* 2 dogs administered CERENIA were not observed on Day 0. Their vomiting status was unknown. 143 was used in the denominator for % vomited.
In US field studies in veterinary patients, CERENIA Injectable Solution and Tablets were well tolerated in dogs presenting with various clinical conditions including parvovirus, gastroenteritis, and renal disease. There were no notable differences in mean laboratory values between CERENIA-treated and placebo-treated patients.
CERENIA Injectable Solution was used safely in dogs receiving other frequently used veterinary products such as fluid and electrolyte replacement solutions, antimicrobial agents, vaccines, antacids, and antiparasitic agents.
In a laboratory study, thirty-one dogs were subcutaneously administered CERENIA Injectable Solution or saline, at 1 mL/10 kg body weight, 45 minutes prior to administration of an opioid analgesic. Following administration of the opioid analgesic, none of the CERENIA Injectable Solution treated dogs vomited and 93.8% (15/16) of placebo-treated dogs vomited.
The effectiveness of CERENIA administered at 1 mg/kg IV was demonstrated by bridging the results of a PK study to clinical data supporting effectiveness of 1 mg/kg administered SC. The IV and SC administration of a single dose of 1 mg/kg maropitant are equivalent, based on the bioequivalence of the IV and SC AUClast and justification for the therapeutic equivalence of the IV and SC Cmax.
CATS
In a field study, 195 cats were presented to veterinary hospitals with a history of vomiting associated with various clinical conditions including gastroenteritis, gastritis, pancreatitis, inflammatory bowel disease, neoplasia, and hepatic lipidosis. Cats were treated with CERENIA Injectable Solution or placebo (in a ratio of 2:1) and observed in the veterinary hospital for 24 hours for the presence of an emetic event(s) defined as the observation of the act of vomiting or the presence of vomitus. Cats could continue antiemetic treatment every 24 hours for up to 5 consecutive days at the discretion of the clinician. Of 165 cats included in the analysis for effectiveness, 2 CERENIA-treated cats (1.8%) vomited 1 time each and 10 placebo-treated cats (18.5%) vomited a total of 15 times in the first 24 hours post treatment.
|
Percent of Cats Vomiting for Each Study Day by Treatment |
||||
|
Study Day |
Treatment |
# cats |
# vomited |
% vomited |
|
Day 0 |
Placebo |
54 |
10 |
18.5 |
|
CERENIA |
111 |
2 |
1.8 |
|
|
Day 1 |
Placebo |
20 |
4 |
20.0 |
|
CERENIA |
34 |
1 |
2.9 |
|
|
Day 2 |
Placebo |
9 |
2 |
22.2 |
|
CERENIA |
8 |
0 |
0 |
|
|
Day 3 |
Placebo |
5 |
0 |
0 |
|
CERENIA |
5 |
0 |
0 |
|
|
Day 4 |
Placebo |
3 |
0 |
0 |
|
CERENIA |
1 |
0 |
0 |
|
The effectiveness of CERENIA administered at 1 mg/kg IV was demonstrated by bridging the results of a PK study to clinical data supporting effectiveness of 1 mg/kg administered SC. The IV and SC administration of a single dose of 1 mg/kg maropitant are equivalent, based on the bioequivalence of the IV and SC AUClast and justification for the therapeutic equivalence of the IV and SC Cmax
ANIMAL SAFETY
DOGS
Laboratory and field studies have demonstrated that CERENIA Injectable Solution is well tolerated in dogs after subcutaneous administration.
Fifty six Beagle dogs (28 males and 28 females) approximately 16 weeks of age were administered CERENIA Injectable Solution subcutaneously once daily for 15 days at 0, 1, 3, and 5 mg/kg. There were 8 dogs (4 males and 4 females) in the 1 mg/kg group and 16 dogs (8 males and 8 females) in all other groups. The primary treatment-related findings were injection site reactions. Swelling, thickened skin, or pain at one or more of the injection sites on one or more days of the study were observed in 6 of 16 animals treated with 3 mg/kg/day and 5 of 16 animals treated with 5 mg/kg/day. Additionally, the activated partial thromboplastin time (APTT) was prolonged (67.5 seconds, reference range 9-15 seconds) in one male dog in the 1 mg/kg group on study day 15. Relationship of the prolonged APTT to drug administration could not be determined.
Beagle dogs approximately 8 weeks of age were administered CERENIA Injectable Solution subcutaneously once daily for 15 days at 0, 1, 3, and 5 mg/kg using a protocol similar to the previous study. A dose dependent increase in frequency and severity of bone marrow hypoplasia was observed histologically. One placebo-treated dog died on day 14 of the study and was diagnosed with suppurative pancreatitis and esophagitis. Interpretation of the study results is complicated by the health status of study animals. Dogs used in the study were weaned early, minimally acclimated to the test facility, and many of the dogs in the study tested positive for coccidia.
Beagle dogs approximately 10 weeks of age were administered either placebo tablets for 2 days, CERENIA Tablets at 8 mg/kg for 2 days, placebo (saline) subcutaneously (SC) for 5 days, CERENIA Injectable Solution at 1 mg/kg SC for 5 days, or CERENIA Tablets at 2 mg/kg for 5 days (8 dogs in each dose group). Mild pain associated with injection was noted in more dogs and lasted longer in dogs that received maropitant injections compared to saline. Males administered CERENIA at 8 mg/kg orally for 2 days had a decrease in food consumption. Body weight and food consumption were variable throughout the 4 week acclimatization period. Two dogs that received 8 mg/kg maropitant orally for 2 days were below the reference range for reticulocyte counts. Decreases in reticulocyte counts were also seen in 4 (of 8) placebo treated dogs (SC saline for 5 days). Hypocellular femoral bone marrow described as "minimal" was seen in 1 male that received 1 mg/kg maropitant SC for 5 days; reticulocyte counts were not available for this dog.
Twenty four Beagle dogs approximately 16 weeks of age were administered CERENIA Injectable Solution intravenously once daily for 5 days at 0, 1, and 3 mg/kg (4 females and 4 males per group). CERENIA Injectable Solution was administered at room temperature over 1-2 minutes. Reaction to injection was not specifically recorded. One male dog in the 1 mg/kg group had low hematocrit and white blood cell count on study day 5. One female dog in the 3 mg/kg group had an increased fibrinogen on study day 5. There were no other clinically relevant findings during the study, at necropsy or in histopathology.
CATS
Thirty-two domestic short hair cats (16 males and 16 females) approximately 16 weeks of age were administered CERENIA Injectable Solution subcutaneously once daily for 15 days at 0, 1, 3, and 5 mg/kg. There were 8 cats (4 males and 4 females) in each group. Treatment-related, dose dependent findings included pain associated with injection of CERENIA and injection site heat, pain, redness, and firmness. Pain on injection was observed in 5% of cats at 0 mg/kg, 50% of cats at 1 mg/kg, and 75% of cats at 3 and 5 mg/kg. Injection site firmness >10 mm in diameter was observed at one or more of the injection sites, on one or more days of the study, in 1 of 8 cats at 1 mg/kg, 7 of 8 cats at 3 mg/kg, and 7 of 8 cats at 5 mg/kg. There was a statistically significant reduction (p=0.0171) in food intake at 5 mg/kg compared to cats at 0 mg/kg. One cat at 5 mg/kg was lethargic on Days 12, 13, and 14 of the study. Increased skin turgor was observed in 1 cat at 3 mg/kg on Days 10 and 11, 1 cat at 3 mg/kg on Day 12, and 1 cat at 5 mg/kg on Day 12. At gross necropsy, there were no treatment-related findings. Histopathologic evaluation of injection sites revealed a dose dependent inflammatory response.
Twenty-four healthy domestic shorthair cats (12 males and 12 females) approximately 16 weeks of age were administered maropitant at 1 or 3 mg/kg, or saline at 0.1 mL/kg intravenously once daily for 5 days. CERENIA Injectable Solution was administered at room temperature over 1-2 minutes. Reaction to injection was not specifically recorded, but one cat experienced discomfort with accidental extravascular administration. There were no clinically relevant findings during the study, at necropsy or in histopathology.
STORAGE CONDITIONS
CERENIA Injectable Solution should be stored at or below 30°C (86°F), with excursions permitted up to 40°C (104°F). After first vial puncture, CERENIA Injectable Solution should be stored at refrigerated temperature 2-8°C (36-46°F). Use within 90 days of first vial puncture. Stopper may be punctured a maximum of 25 times.
HOW SUPPLIED
CERENIA Injectable Solution is supplied in 20 mL amber glass vials. Each mL contains 10 mg of maropitant as maropitant citrate.
REFERENCES
1. Diemunsch P, Grelot L. Potential of substance P antagonists as antiemetics. [Review] [60 refs]. Drugs. 2000;60:533-46.
