PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
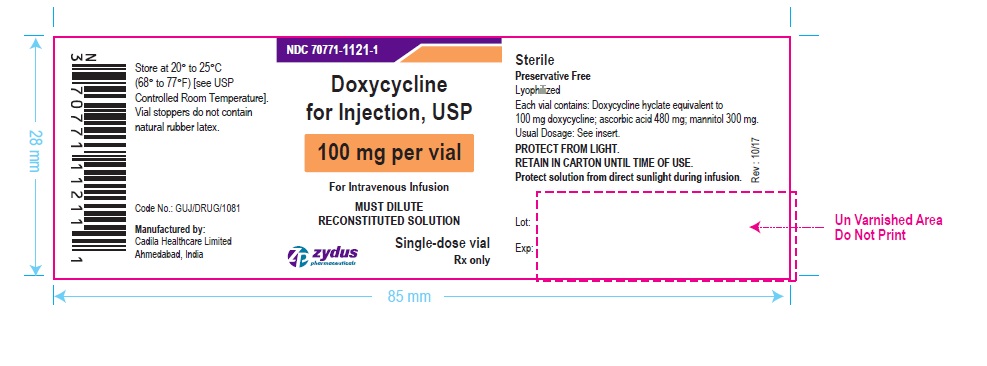
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL – DOXYCYCLINE 100 MG CONTAINER LABEL
NDC 70771-1121-1
Doxycycline for Injection, USP
100 mg per vial
For Intravenous Infusion
MUST DILUTE RECONSTITUTED SOLUTION
Single-dose vial
Rx only
Zydus Pharmaceuticals
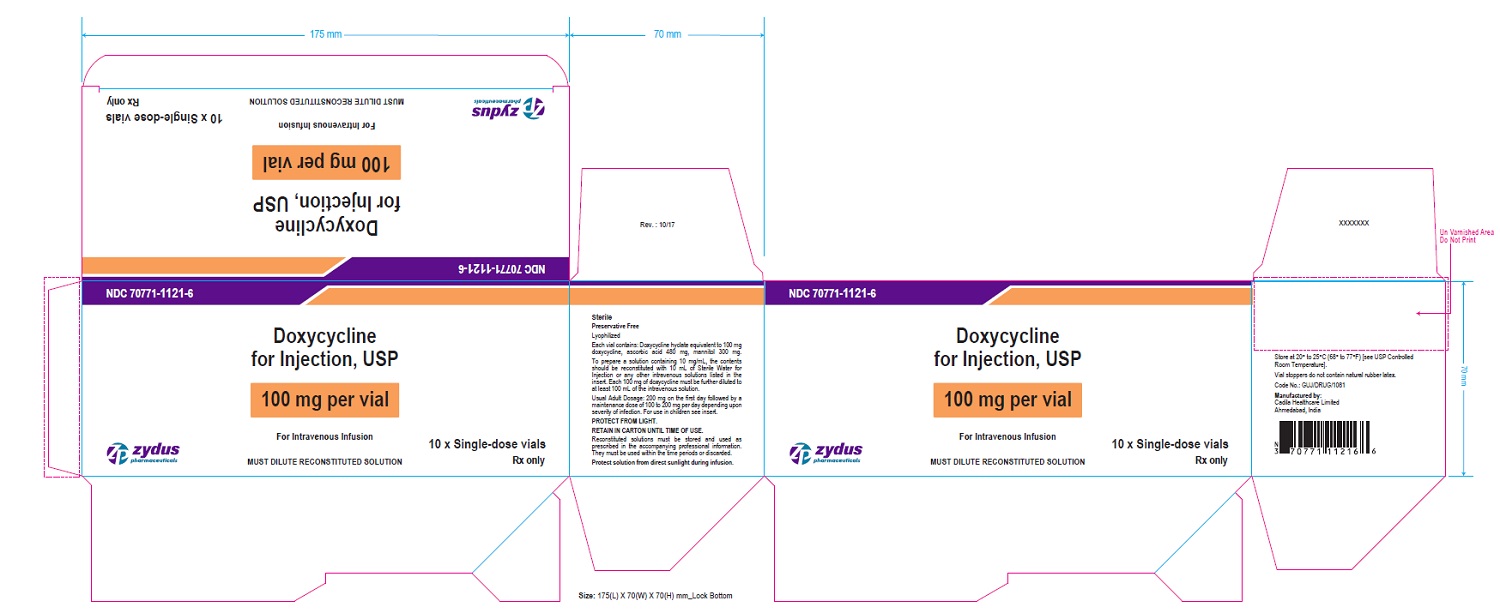
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - DOXYCYCLINE 100 MG CARTON LABEL
NDC 70771-1121-6
Doxycycline for Injection, USP
100 mg per vial
For Intravenous Infusion
MUST DILUTE RECONSTITUTED SOLUTION
10 x Single-dose vials
Rx only
Zydus Pharmaceuticals
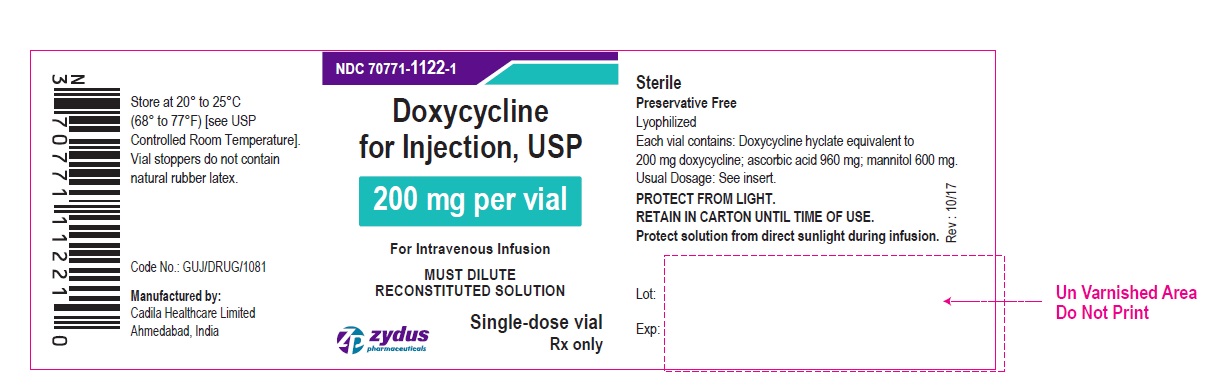
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL – DOXYCYCLINE 200 MG CONTAINER LABEL
NDC 70771-1122-1
Doxycycline for Injection, USP
200 mg per vial
For Intravenous Infusion
MUST DILUTE RECONSTITUTED SOLUTION
Single-dose vial
Rx only
Zydus Pharmaceuticals
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - DOXYCYCLINE 200 MG CARTON LABEL
NDC 70771-1122-1
Doxycycline for Injection, USP
200 mg per vial
For Intravenous Infusion
MUST DILUTE RECONSTITUTED SOLUTION
Single-dose vial
Rx only
Zydus Pharmaceuticals
