FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Indication
SOLODYN is indicated to treat only inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 12 years of age and older.
1.2 Limitations of Use
SOLODYN did not demonstrate any effect on non-inflammatory acne lesions. Safety of SOLODYN has not been established beyond 12 weeks of use. This formulation of minocycline has not been evaluated in the treatment of infections [see Clinical Studies (14)].
To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, SOLODYN should be used only as indicated [see Warnings and Precautions (5.11)].
2 DOSAGE AND ADMINISTRATION
The recommended dosage of SOLODYN is approximately 1 mg/kg once daily for 12 weeks. Higher doses have not shown to be of additional benefit in the treatment of inflammatory lesions of acne, and may be associated with more acute vestibular side effects.
The following table shows tablet strength and body weight to achieve approximately 1 mg/kg.
| Patient's Weight (lbs.) | Patient's Weight (kg) | Tablet Strength (mg) | Actual mg/kg Dose |
|---|---|---|---|
| 99 – 109 | 45 – 49 | 45 | 1 – 0.92 |
| 110 – 131 | 50 – 59 | 55 | 1.10 – 0.93 |
| 132 – 157 | 60 – 71 | 65 | 1.08 – 0.92 |
| 158 – 186 | 72 – 84 | 80 | 1.11 – 0.95 |
| 187 – 212 | 85 – 96 | 90 | 1.06 – 0.94 |
| 213 – 243 | 97 – 110 | 105 | 1.08 – 0.95 |
| 244 – 276 | 111 – 125 | 115 | 1.04 – 0.92 |
| 277 – 300 | 126 – 136 | 135 | 1.07 – 0.99 |
SOLODYN Tablets may be taken with or without food [see Clinical Pharmacology (12)]. Ingestion of food along with SOLODYN may help reduce the risk of esophageal irritation and ulceration.
In patients with renal impairment, the total dosage should be decreased by either reducing the recommended individual doses and/or by extending the time intervals between doses [see Warnings and Precautions (5.4)].
3 DOSAGE FORMS AND STRENGTHS
- 45 mg extended release tablets: gray, unscored, coated, and debossed with "DYN-045" on one side.
- 55 mg extended release tablets: pink, unscored, coated, and debossed with "DYN-055" on one side.
- 65 mg extended release tablets: blue, unscored, coated, and debossed with "DYN-065" on one side.
- 80 mg extended release tablets: gray, unscored, coated, and debossed with "DYN-080" on one side.
- 90 mg extended release tablets: yellow, unscored, coated, and debossed with "DYN-090" on one side.
- 105 mg extended release tablets: purple, unscored, coated, and debossed with "DYN-105" on one side.
- 115 mg extended release tablets: green, unscored, coated, and debossed with "DYN-115" on one side.
- 135 mg extended release tablets: pink (orange-brown), unscored, coated, and debossed with "DYN-135" on one side.
4 CONTRAINDICATIONS
This drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
5 WARNINGS AND PRECAUTIONS
5.1 Teratogenic Effects
- MINOCYCLINE, LIKE OTHER TETRACYCLINE-CLASS DRUGS, CAN CAUSE FETAL HARM WHEN ADMINISTERED TO A PREGNANT WOMAN. IF ANY TETRACYCLINE IS USED DURING PREGNANCY OR IF THE PATIENT BECOMES PREGNANT WHILE TAKING THESE DRUGS, THE PATIENT SHOULD BE APPRISED OF THE POTENTIAL HAZARD TO THE FETUS.
SOLODYN should not be used during pregnancy or by individuals of either gender who are attempting to conceive a child [see Nonclinical Toxicology (13.1) & Use in Specific Populations (8.1)]. - THE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY, AND CHILDHOOD UP TO THE AGE OF 8 YEARS) MAY CAUSE PERMANENT DISCOLORATION OF THE TEETH (YELLOW-GRAY-BROWN).
This adverse reaction is more common during long-term use of the drug but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. TETRACYCLINE DRUGS, THEREFORE, SHOULD NOT BE USED DURING TOOTH DEVELOPMENT. - All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature human infants given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued.
Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can cause retardation of skeletal development on the developing fetus. Evidence of embryotoxicity has been noted in animals treated early in pregnancy [see Use in Specific Populations (8.1)].
5.2 Pseudomembranous Colitis
Pseudomembranous colitis has been reported with nearly all antibacterial agents and may range from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of "antibiotic-associated colitis".
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
5.3 Hepatotoxicity
Post-marketing cases of serious liver injury, including irreversible drug-induced hepatitis and fulminant hepatic failure (sometimes fatal) have been reported with minocycline use in the treatment of acne.
5.4 Metabolic Effects
The anti-anabolic action of the tetracyclines may cause an increase in BUN. While this is not a problem in those with normal renal function, in patients with significantly impaired function, higher serum levels of tetracycline-class drugs may lead to azotemia, hyperphosphatemia, and acidosis. If renal impairment exists, even usual oral or parenteral doses may lead to excessive systemic accumulations of the drug and possible liver toxicity. Under such conditions, lower than usual total doses are indicated, and if therapy is prolonged, serum level determinations of the drug may be advisable.
5.5 Central Nervous System Effects
Central nervous system side effects including light-headedness, dizziness or vertigo have been reported with minocycline therapy. Patients who experience these symptoms should be cautioned about driving vehicles or using hazardous machinery while on minocycline therapy. These symptoms may disappear during therapy and usually rapidly disappear when the drug is discontinued.
5.6 Benign Intracranial Hypertension
Pseudotumor cerebri (benign intracranial hypertension) in adults and adolescents has been associated with the use of tetracyclines. Minocycline has been reported to cause or precipitate pseudotumor cerebri, the hallmark of which is papilledema. Clinical manifestations include headache and blurred vision. Bulging fontanels have been associated with the use of tetracyclines in infants. Although signs and symptoms of pseudotumor cerebri resolve after discontinuation of treatment, the possibility for permanent sequelae such as visual loss that may be permanent or severe exists. Patients should be questioned for visual disturbances prior to initiation of treatment with tetracyclines. If visual disturbance occurs during treatment, patients should be checked for papilledema. Concomitant use of isotretinoin and minocycline should be avoided because isotretinoin, a systemic retinoid, is also known to cause pseudotumor cerebri.
5.7 Autoimmune Syndromes
Tetracyclines have been associated with the development of autoimmune syndromes. The long-term use of minocycline in the treatment of acne has been associated with drug-induced lupus-like syndrome, autoimmune hepatitis and vasculitis. Sporadic cases of serum sickness have presented shortly after minocycline use. Symptoms may be manifested by fever, rash, arthralgia, and malaise. In symptomatic patients, liver function tests, ANA, CBC, and other appropriate tests should be performed to evaluate the patients. Use of all tetracycline-class drugs should be discontinued immediately.
5.8 Photosensitivity
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. This has been reported rarely with minocycline. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using minocycline. If patients need to be outdoors while using minocycline, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician.
5.9 Serious Skin/Hypersensitivity Reaction
Cases of anaphylaxis, serious skin reactions (e.g. Stevens Johnson syndrome), erythema multiforme, and drug rash with eosinophilia and systemic symptoms (DRESS) syndrome have been reported postmarketing with minocycline use in patients with acne. DRESS syndrome consists of cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, and one or more of the following visceral complications such as: hepatitis, pneumonitis, nephritis, myocarditis, and pericarditis. Fever and lymphadenopathy may be present. In some cases, death has been reported. If this syndrome is recognized, the drug should be discontinued immediately.
5.10 Tissue Hyperpigmentation
Tetracycline-class antibiotics are known to cause hyperpigmentation. Tetracycline therapy may induce hyperpigmentation in many organs, including nails, bone, skin, eyes, thyroid, visceral tissue, oral cavity (teeth, mucosa, alveolar bone), sclerae and heart valves. Skin and oral pigmentation has been reported to occur independently of time or amount of drug administration, whereas other tissue pigmentation has been reported to occur upon prolonged administration. Skin pigmentation includes diffuse pigmentation as well as over sites of scars or injury.
5.11 Development of Drug-Resistant Bacteria
Bacterial resistance to the tetracyclines may develop in patients using SOLODYN, therefore, the susceptibility of bacteria associated with infection should be considered in selecting antimicrobial therapy. Because of the potential for drug-resistant bacteria to develop during the use of SOLODYN, it should be used only as indicated.
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under prescribed conditions, adverse reaction rates observed in the clinical trial may not reflect the rates observed in practice.
The following table summarizes selected adverse reactions reported in clinical trials at a rate of ≥ 1% for SOLODYN.
| Adverse Reactions | SOLODYN (1 mg/kg) N = 674 (%) | PLACEBO N = 364 (%) |
|---|---|---|
| At least one treatment-emergent event | 379 (56) | 197 (54) |
| Headache | 152 (23) | 83 (23) |
| Fatigue | 62 (9) | 24 (7) |
| Dizziness | 59 (9) | 17 (5) |
| Pruritus | 31 (5) | 16 (4) |
| Malaise | 26 (4) | 9 (3) |
| Mood alteration | 17 (3) | 9 (3) |
| Somnolence | 13 (2) | 3 (1) |
| Urticaria | 10 (2) | 1 (0) |
| Tinnitus | 10 (2) | 5 (1) |
| Arthralgia | 9 (1) | 2 (0) |
| Vertigo | 8 (1) | 3 (1) |
| Dry mouth | 7 (1) | 5 (1) |
| Myalgia | 7 (1) | 4 (1) |
6.2 Postmarketing Experience
Adverse reactions that have been reported with minocycline hydrochloride use in a variety of indications include:
- Skin and hypersensitivity reactions: fixed drug eruptions, balanitis, erythema multiforme, Stevens-Johnson syndrome, anaphylactoid purpura, photosensitivity, pigmentation of skin and mucous membranes, hypersensitivity reactions, angioneurotic edema, anaphylaxis, DRESS syndrome [see Warnings and Precautions (5.9)].
- Autoimmune conditions: polyarthralgia, pericarditis, exacerbation of systemic lupus, pulmonary infiltrates with eosinophilia, transient lupus-like syndrome.
- Central nervous system: pseudotumor cerebri, bulging fontanels in infants, decreased hearing.
- Endocrine: brown-black microscopic thyroid discoloration, abnormal thyroid function.
- Oncology: thyroid cancer.
- Oral: glossitis, dysphagia, tooth discoloration.
- Gastrointestinal: enterocolitis, pancreatitis, hepatitis, liver failure.
- Renal: reversible acute renal failure.
- Hematology: hemolytic anemia, thrombocytopenia, eosinophilia.
Preliminary studies suggest that use of minocycline may have deleterious effects on human spermatogenesis [see Nonclinical Toxicology (13.1)].
7 DRUG INTERACTIONS
7.1 Anticoagulants
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
7.2 Penicillin
Since bacteriostatic drugs may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracycline-class drugs in conjunction with penicillin.
7.3 Methoxyflurane
The concurrent use of tetracycline and methoxyflurane has been reported to result in fatal renal toxicity.
7.4 Antacids and Iron Preparations
Absorption of tetracyclines is impaired by antacids containing aluminum, calcium or magnesium and iron-containing preparations.
7.5 Low Dose Oral Contraceptives
In a multi-center study to evaluate the effect of SOLODYN on low dose oral contraceptives, hormone levels over one menstrual cycle with and without SOLODYN 1 mg/kg once-daily were measured. Based on the results of this trial, minocycline-related changes in estradiol, progestinic hormone, FSH and LH plasma levels, of breakthrough bleeding, or of contraceptive failure, can not be ruled out. To avoid contraceptive failure, female patients are advised to use a second form of contraceptive during treatment with minocycline.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy category D [see Warnings and Precautions (5.1)]
SOLODYN should not be used during pregnancy. If the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus and stop treatment immediately.
There are no adequate and well-controlled studies on the use of minocycline in pregnant women. Minocycline, like other tetracycline-class drugs, crosses the placenta and may cause fetal harm when administered to a pregnant woman.
Rare spontaneous reports of congenital anomalies including limb reduction have been reported with minocycline use in pregnancy in post-marketing experience. Only limited information is available regarding these reports; therefore, no conclusion on causal association can be established.
Minocycline induced skeletal malformations (bent limb bones) in fetuses when administered to pregnant rats and rabbits in doses of 30 mg/kg/day and 100 mg/kg/day, respectively, (resulting in approximately 3 times and 2 times, respectively, the systemic exposure to minocycline observed in patients as a result of use of SOLODYN). Reduced mean fetal body weight was observed in studies in which minocycline was administered to pregnant rats at a dose of 10 mg/kg/day (which resulted in approximately the same level of systemic exposure to minocycline as that observed in patients who use SOLODYN).
Minocycline was assessed for effects on peri- and post-natal development of rats in a study that involved oral administration to pregnant rats from day 6 of gestation through the period of lactation (postpartum day 20), at dosages of 5, 10, or 50 mg/kg/day. In this study, body weight gain was significantly reduced in pregnant females that received 50 mg/kg/day (resulting in approximately 2.5 times the systemic exposure to minocycline observed in patients as a result of use of SOLODYN). No effects of treatment on the duration of the gestation period or the number of live pups born per litter were observed. Gross external anomalies observed in F1 pups (offspring of animals that received minocycline) included reduced body size, improperly rotated forelimbs, and reduced size of extremities. No effects were observed on the physical development, behavior, learning ability, or reproduction of F1 pups, and there was no effect on gross appearance of F2 pups (offspring of F1 animals).
8.3 Nursing Mothers
Tetracycline-class antibiotics are excreted in human milk. Because of the potential for serious adverse effects on bone and tooth development in nursing infants from the tetracycline-class antibiotics, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother [see Warnings and Precautions (5.1)].
8.4 Pediatric Use
SOLODYN is indicated to treat only inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 12 years and older. Safety and effectiveness in pediatric patients below the age of 12 has not been established.
Use of tetracycline-class antibiotics below the age of 8 is not recommended due to the potential for tooth discoloration [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Clinical studies of SOLODYN did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy.
10 OVERDOSAGE
In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Minocycline is not removed in significant quantities by hemodialysis or peritoneal dialysis.
11 DESCRIPTION
Minocycline hydrochloride, a semi synthetic derivative of tetracycline, is [4S-(4α,4aα,5aα,12aα)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide mono hydrochloride. The structural formula is represented below:
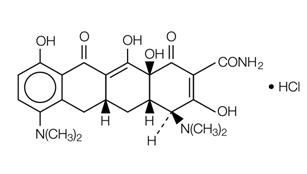
C23H27N3O7•HCl M. W. 493.95
SOLODYN Tablets for oral administration contain minocycline hydrochloride USP equivalent to 45 mg, 55 mg, 65 mg, 80 mg, 90 mg, 105 mg, 115 mg, or 135 mg of minocycline. In addition, 45 mg, 55 mg, 65 mg, 80 mg, 90 mg, 105 mg, 115 mg, and 135 mg tablets contain the following inactive ingredients: lactose monohydrate NF, hypromellose type 2910 USP, magnesium stearate NF, colloidal silicon dioxide NF, and carnauba wax NF. The 45 mg tablets also contain Opadry II Gray which contains: lactose monohydrate NF, hypromellose type 2910 USP, titanium dioxide USP, triacetin USP, and iron oxide black JPE. The 55 mg tablets also contain Opadry II Pink which contains: hypromellose type 2910 USP, titanium dioxide USP, lactose monohydrate NF, polyethylene glycol 3350 NF, triacetin USP, and FD&C Red #40. The 65 mg tablets also contain Opadry II Blue which contains: hypromellose type 2910 USP, lactose monohydrate NF, FD&C Blue #1, polyethylene glycol 3350 NF, FD&C Blue #2, titanium dioxide USP, triacetin USP, and D&C Yellow #10. The 80 mg tablets also contain Opadry II Gray which contains: hypromellose type 2910 USP, lactose monohydrate NF, polyethylene glycol 3350 NF, FD&C Blue #2, FD&C Red #40, titanium dioxide USP, triacetin USP, and FD&C Yellow #6. The 90 mg tablets also contain Opadry II Yellow which contains: hypromellose type 2910 USP, lactose monohydrate NF, titanium dioxide USP, iron oxide yellow NF, polyethylene glycol 3350 NF, and triacetin USP. The 105 mg tablets also contain Opadry II Purple which contains: hypromellose type 2910 USP, lactose monohydrate NF, titanium dioxide USP, D&C Red #27, polyethylene glycol 3350 NF, triacetin USP, and FD&C Blue #1. The 115 mg tablets also contain Opadry II Green which contains: hypromellose type 2910 USP, lactose monohydrate NF, D&C Yellow #10, triacetin USP, FD&C Blue #1, titanium dioxide USP, and FD&C Blue #2. The 135 mg tablets also contain Opadry II Pink which contains: hypromellose type 2910 USP, lactose monohydrate NF, titanium dioxide USP, polyethylene glycol 3350 NF, iron oxide red NF, and triacetin USP.
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
SOLODYN Tablets are not bioequivalent to non-modified release minocycline products. Based on pharmacokinetic studies in healthy adults, SOLODYN Tablets produce a delayed Tmax at 3.5–4.0 hours as compared to a non-modified release reference minocycline product (Tmax at 2.25–3 hours). At steady-state (Day 6), the mean AUC(0–24) and Cmax were 33.32 µg×hr/mL and 2.63 µg/mL for SOLODYN Tablets and 46.35 µg×hr/mL and 2.92 µg/mL for Minocin® capsules, respectively. These parameters are based on dose adjusted to 135 mg per day for both products.
A single-dose, four-way crossover study demonstrated that SOLODYN Tablets used in the study (45 mg, 90 mg, 135 mg) exhibited dose-proportional pharmacokinetics. In another single-dose, five-way crossover pharmacokinetic study, SOLODYN Tablets 55 mg, 80 mg, and 105 mg were shown to be dose-proportional to SOLODYN Tablets 90 mg and 135 mg.
When SOLODYN Tablets were administered concomitantly with a meal that included dairy products, the extent and timing of absorption of minocycline did not differ from that of administration under fasting conditions.
Minocycline is lipid soluble and distributes into the skin and sebum.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis—Long-term animal studies have not been performed to evaluate the carcinogenic potential of minocycline. A structurally related compound, oxytetracycline, was found to produce adrenal and pituitary tumors in rats.
Mutagenesis—Minocycline was not mutagenic in vitro in a bacterial reverse mutation assay (Ames test) or CHO/HGPRT mammalian cell assay in the presence or absence of metabolic activation. Minocycline was not clastogenic in vitro using human peripheral blood lymphocytes or in vivo in a mouse micronucleus test.
Impairment of Fertility—Male and female reproductive performance in rats was unaffected by oral doses of minocycline of up to 300 mg/kg/day (which resulted in up to approximately 40 times the level of systemic exposure to minocycline observed in patients as a result of use of SOLODYN). However, oral administration of 100 or 300 mg/kg/day of minocycline to male rats (resulting in approximately 15 to 40 times the level of systemic exposure to minocycline observed in patients as a result of use of SOLODYN) adversely affected spermatogenesis. Effects observed at 300 mg/kg/day included a reduced number of sperm cells per gram of epididymis, an apparent reduction in the percentage of sperm that were motile, and (at 100 and 300 mg/kg/day) increased numbers of morphologically abnormal sperm cells. Morphological abnormalities observed in sperm samples included absent heads, misshapen heads, and abnormal flagella.
Limited human studies suggest that minocycline may have a deleterious effect on spermatogenesis.
SOLODYN should not be used by individuals of either gender who are attempting to conceive a child.
14 CLINICAL STUDIES
The safety and efficacy of SOLODYN in the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris was assessed in two 12-week, multi-center, randomized, double-blind, placebo-controlled, studies in subjects ≥ 12 years. The mean age of subjects was 20 years and subjects were from the following racial groups: White (73%), Hispanic (13%), Black (11%), Asian/Pacific Islander (2%), and Other (2%).
In two efficacy and safety trials, a total of 924 subjects with non-nodular moderate to severe acne vulgaris received SOLODYN or placebo for a total of 12 weeks, according to the following dose assignments.
| Subject's Weight (lbs.) | Subject's Weight (kg) | Available Caplet Strength (mg) | Actual mg/kg Dose |
|---|---|---|---|
| 99 – 131 | 45 – 59 | 45 | 1 – 0.76 |
| 132 – 199 | 60 – 90 | 90 | 1.5 – 1 |
| 200 – 300 | 91 – 136 | 135 | 1.48 – 0.99 |
The two primary efficacy endpoints were:
- 1)
- Mean percent change in inflammatory lesion counts from Baseline to 12 weeks.
- 2)
- Percentage of subjects with an Evaluator's Global Severity Assessment (EGSA) of clear or almost clear at 12 weeks.
Efficacy results are presented in Table 4.
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| SOLODYN (1 mg/kg) N = 300 | Placebo N = 151 | SOLODYN (1 mg/kg) N = 315 | Placebo N = 158 |
|
| Mean Percent Improvement in Inflammatory Lesions | 43.1% | 31.7% | 45.8% | 30.8% |
| No. (%) of Subjects Clear or Almost Clear on the EGSA | 52 (17.3%) | 12 (7.9%) | 50 (15.9%) | 15 (9.5%) |
SOLODYN did not demonstrate any effect on non-inflammatory lesions (benefit or worsening).
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
SOLODYN (minocycline HCl, USP) Extended Release Tablets are supplied as aqueous film coated tablets containing minocycline hydrochloride equivalent to 80 mg minocycline, are supplied as follows.
The 80 mg extended release tablets are gray, unscored, coated, and debossed with "DYN-080" on one side. Each tablet contains minocycline hydrochloride equivalent to 80 mg minocycline, supplied as follows:
| NDC 54868-6285-0 | Bottle of 30 |
17 PATIENT COUNSELING INFORMATION
[See FDA-approved patient labeling (Patient Information)]
Patients taking SOLODYN (minocycline HCl, USP) Extended Release Tablets should receive the following information and instructions:
- SOLODYN should not be used by pregnant women or women attempting to conceive a child [see Use in Specific Populations (8.1), Nonclinical Toxicology (13.1)].
- It is recommended that SOLODYN not be used by men who are attempting to father a child [see Nonclinical Toxicology (13.1)].
- Patients should be advised that pseudomembranous colitis can occur with minocycline therapy. If patients develop watery or bloody stools, they should seek medical attention.
- Patients should be counseled about the possibility of hepatotoxicity. Patients should seek medical advice if they experience symptoms which can include loss of appetite, tiredness, diarrhea, skin turning yellow, bleeding easily, confusion, and sleepiness.
- Patients who experience central nervous system symptoms [see Warnings and Precautions (5.5)] should be cautioned about driving vehicles or using hazardous machinery while on minocycline therapy. Patients should seek medical help for persistent headaches or blurred vision.
- Concurrent use of tetracycline may render oral contraceptives less effective [see Drug Interactions (7.5)].
- Autoimmune syndromes, including drug-induced lupus-like syndrome, autoimmune hepatitis, vasculitis and serum sickness have been observed with tetracycline-class drugs, including minocycline. Symptoms may be manifested by arthralgia, fever, rash and malaise. Patients who experience such symptoms should be cautioned to stop the drug immediately and seek medical help.
- Patients should be counseled about discoloration of skin, scars, teeth or gums that can arise from minocycline therapy.
- Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines, including minocycline. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using minocycline. If patients need to be outdoors while using minocycline, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. Treatment should be discontinued at the first evidence of skin erythema.
- SOLODYN should be taken exactly as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the current treatment course and increase the likelihood that bacteria will develop resistance and will not be treatable by other antibacterial drugs in the future.
- Patients should be advised to swallow SOLODYN Tablets whole and not to chew, crush, or split the tablets.
Patient Information
SOLODYN® (SO-lo-dīn)
(minocycline HCl)
Extended Release Tablets
Read this Patient Information leaflet that comes with SOLODYN before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or treatment.
What is SOLODYN?
SOLODYN is a tetracycline-class drug. SOLODYN is prescription medicine used to treat pimples and red bumps (non-nodular inflammatory lesions) that happen with moderate to severe acne vulgaris in people 12 years and older. SOLODYN is not effective for acne that is not red-looking (this means acne that is not inflammatory).
It is not known if SOLODYN is:
- safe for use longer than 12 weeks.
- safe and effective for the treatment of infections.
- safe and effective in children under the age of 12 years.
Who should not take SOLODYN?
Do not take SOLODYN if you are allergic to tetracycline-class drugs. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
What should I tell my doctor before taking SOLODYN?
Before you take SOLODYN, tell your doctor if you:
- have kidney problems. Your doctor may prescribe a lower dose of medicine for you.
- have liver problems.
- have diarrhea or watery stools.
- have vision problems.
- plan to have surgery with general anesthesia.
- have any other medical conditions.
- are a male, and you and your female partner are trying to conceive a baby. You should not take SOLODYN\.
- are pregnant or plan to become pregnant. SOLODYN may harm your unborn baby. Taking SOLODYN while you are pregnant may cause serious side effects on the growth of bone and teeth of your baby. Talk to your doctor before taking SOLODYN if you plan to become pregnant, or if you are already taking SOLODYN and plan to become pregnant. Stop taking SOLODYN and call your doctor right away if you become pregnant while taking SOLODYN.
- are breastfeeding or plan to breastfeed. SOLODYN passes into your milk and may harm your baby. You and your doctor should decide if you will take SOLODYN or breastfeed. You should not do both.
Tell your doctor about all the other medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. SOLODYN® may affect the way other medicines work, and other medicines may affect how SOLODYN® works.
Especially tell your doctor if you take:
- birth control pills. SOLODYN® may make your birth control pills less effective. You could become pregnant. You should use a second form of birth control while taking SOLODYN®.
- a blood thinner medicine.
- a penicillin antibiotic medicine. SOLODYN® and penicillins should not be used together.
- antacids that contain aluminum, calcium, or magnesium or iron-containing products.
- an acne medicine that contains isotretinoin (Amnesteem, Claravis, Sotret). SOLODYN® and isotretinoin should not be used together.
Ask your doctor or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist.
How should I take SOLODYN?
- Take SOLODYN exactly as your doctor tells you.
- Skipping doses or not taking all doses of SOLODYN may:
- make the treatment not work as well.
- increase the chance that the bacteria will become resistant to SOLODYN.
- SOLODYN can be taken with or without food. Taking SOLODYN with food may lower your chances of getting irritation or ulcers in your esophagus. Your esophagus is the tube that connects your mouth to your stomach.
- Swallow SOLODYN Tablets whole. Do not chew, crush, or split the tablets.
If you take too much SOLODYN, call your doctor or poison control center right away. Your doctor may do blood tests to check you for side effects during treatment with SOLODYN.
What should I avoid while taking SOLODYN?
- Avoid sunlight, sunlamps, and tanning beds. SOLODYN can make your skin sensitive to the sun and the light from sunlamps and tanning beds. You could get severe sunburn.
- Protect your skin while out in sunlight.
- You should not drive or operate dangerous machinery until you know how SOLODYN affects you. SOLODYN may cause you to feel dizzy or lightheaded, or have a spinning feeling (vertigo).
What are possible side effects of SOLODYN?
SOLODYN may cause serious side effects, including:
- Harm to an unborn baby. See "What should I tell my doctor before taking SOLODYN?"
- Permanent teeth discoloration. SOLODYN may permanently turn a baby or child's teeth yellow-grey-brown during tooth development. SOLODYN should not be used during tooth development. Tooth development happens in the last half of pregnancy, and from birth to 8 years of age. See "What should I tell my doctor before taking SOLODYN?"
- Intestine infection (pseudomembranous colitis). Pseudomembranous colitis can happen with most antibiotics, including SOLODYN. Call your doctor right away if you get watery diarrhea, diarrhea that does not go away, or bloody stools.
-
Serious liver problems. Stop taking SOLODYN and call your doctor right away if you get any of the following symptoms of liver problems:
- loss of appetite
- tiredness
- diarrhea
- yellowing of your skin or the whites of your eyes
- unexplained bleeding
- confusion
- sleepiness
- Central nervous system effects. See "What should I avoid while taking SOLODYN?" Central nervous system effects such as light headedness, dizziness, and a spinning feeling (vertigo) may go away during your treatment with SOLODYN or if treatment is stopped.
- Benign intracranial hypertension, also called pseudotumor cerebri. This is a condition where there is high pressure in the fluid around the brain. This swelling may lead to vision changes and permanent vision loss. Stop taking SOLODYN and tell your doctor right away if you have blurred vision, vision loss, or unusual headaches.
- Immune system reactions including a lupus-like syndrome, hepatitis, and inflammation of blood or lymph vessels (vasculitis). Using SOLODYN for a long time to treat acne may cause immune system reactions. Tell your doctor right away if you get a fever, rash, joint pain, or body weakness. Your doctor may do tests to check your blood for immune system reactions.
- Serious rash and allergic reactions. SOLODYN may cause a serious rash and allergic reactions that may affect parts of your body such as your liver, lungs, kidneys and heart. Sometimes these can lead to death.
- Stop taking SOLODYN and get medical help right away if you have any of these symptoms:
- skin rash, hives, sores in your mouth, or your skin blisters and peels
- swelling of your face, eyes, lips, tongue, or throat
- trouble swallowing or breathing
- blood in your urine
- fever, yellowing of the skin or the whites of your eyes, dark colored urine
- pain on the right side of the stomach area (abdominal pain)
- chest pain or abnormal heartbeats
- swelling in your legs, ankles, and feet
- darkening of your nails, skin, eyes, scars, teeth, and gums
The most common side effects of SOLODYN include:
- headache
- tiredness
- dizziness or spinning feeling
- itching
Call your doctor if you have a side effect that bothers you or that does not go away. Your doctor may do tests to check you for side effects during treatment with SOLODYN.
These are not all the side effects with SOLODYN. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Medicis at 1-800-900-6389.
How should I store SOLODYN?
- Store SOLODYN between 59°F to 86°F (15°C to 30°C).
- Keep SOLODYN Tablets in the container that it comes in and keep the container tightly closed.
- Keep SOLODYN Tablets dry.
Keep SOLODYN and all medicines out of the reach of children.
General information about SOLODYN
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use SOLODYN for a condition for which it was not prescribed. Do not give SOLODYN to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about SOLODYN. If you would like more information, talk to your doctor. You can ask your doctor or pharmacist for information about SOLODYN that is written for health professionals.
For more information, call 1-800-550-5115.
What are the ingredients in SOLODYN?
Active ingredient: minocycline HCl.
Inactive ingredients: lactose monohydrate, hypromellose type 2910, magnesium stearate, colloidal silicon dioxide, and carnauba wax.
The 45 mg tablets also contain Opadry II Gray which contains: lactose monohydrate, hypromellose type 2910, titanium dioxide, triacetin, and iron oxide black JPE.
The 55 mg tablets also contain Opadry II Pink which contains: hypromellose type 2910, titanium dioxide, lactose monohydrate, polyethylene glycol 3350, triacetin, and FD&C Red #40.
The 65 mg tablets also contain Opadry II Blue which contains: hypromellose type 2910, lactose monohydrate, FD&C Blue #1, polyethylene glycol 3350, FD&C Blue #2, titanium dioxide, triacetin, and D&C Yellow #10.
The 80 mg tablets also contain Opadry II Gray which contains: hypromellose type 2910, lactose monohydrate, polyethylene glycol 3350, FD&C Blue #2, FD&C Red #40, titanium dioxide, triacetin, and FD&C Yellow #6.
The 90 mg tablets also contain Opadry II Yellow which contains: hypromellose type 2910, lactose monohydrate, titanium dioxide, iron oxide yellow, polyethylene glycol 3350, and triacetin.
The 105 mg tablets also contain Opadry II Purple which contains: hypromellose type 2910, lactose monohydrate, titanium dioxide, D&C Red #27, polyethylene glycol 3350, triacetin, and FD&C Blue #1.
The 115 mg tablets also contain Opadry II Green which contains: hypromellose type 2910, lactose monohydrate NF, D&C Yellow #10, triacetin, FD&C Blue #1, titanium dioxide, FD&C Blue #2.
The 135 mg tablets also contain Opadry II Pink which contains: hypromellose type 2910, lactose monohydrate, titanium dioxide, polyethylene glycol 3350, iron oxide red, and triacetin.
SOLODYN® is manufactured by WellSpring Pharmaceutical Canada Corp. for Medicis Pharmaceutical Corporation, Scottsdale, Arizona, 85256.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 03/2011
U.S. Patent 5,908,838, U.S. Patent 7,790,705 and Patents Pending1
© 2010 Medicis Pharmaceutical Corporation
SOLODYN is a registered trademark of Medicis Pharmaceutical Corporation.
All other trademarks are the properties of their respective owners.
Manufactured for:
Medicis, The Dermatology Company
Scottsdale, AZ 85256
Manufactured by:
WellSpring Pharmaceutical Canada Corp.
Oakville, Ontario, CANADA L6H 1M5
N4605D
- 1
- 90 mg is also covered by U.S. Patents 7,541,347 and 7,544,373
