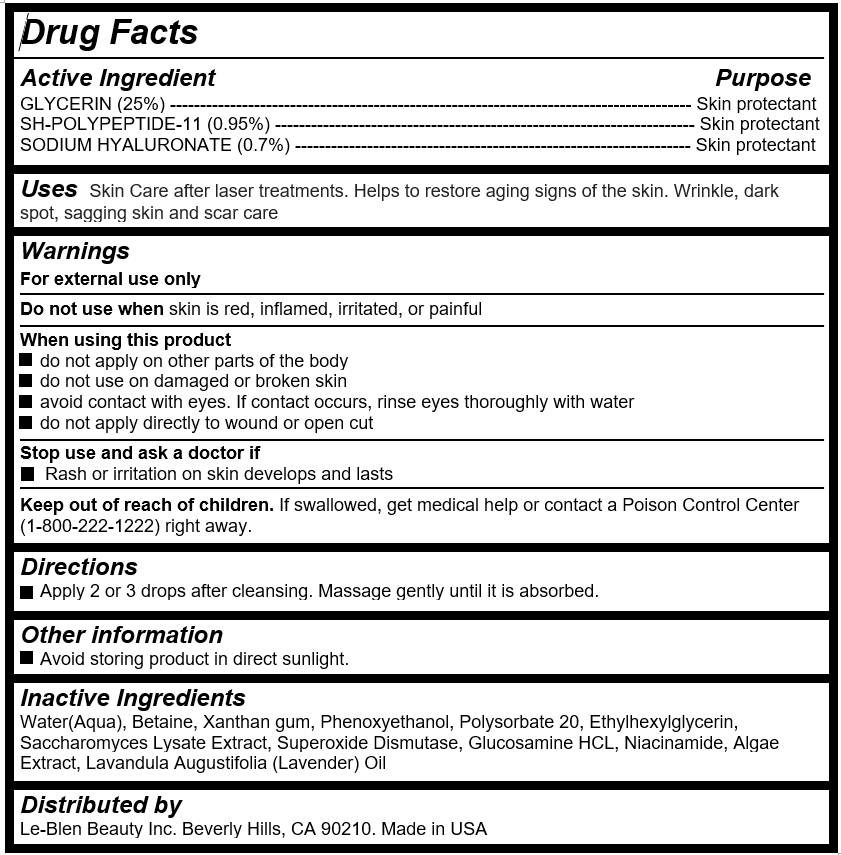
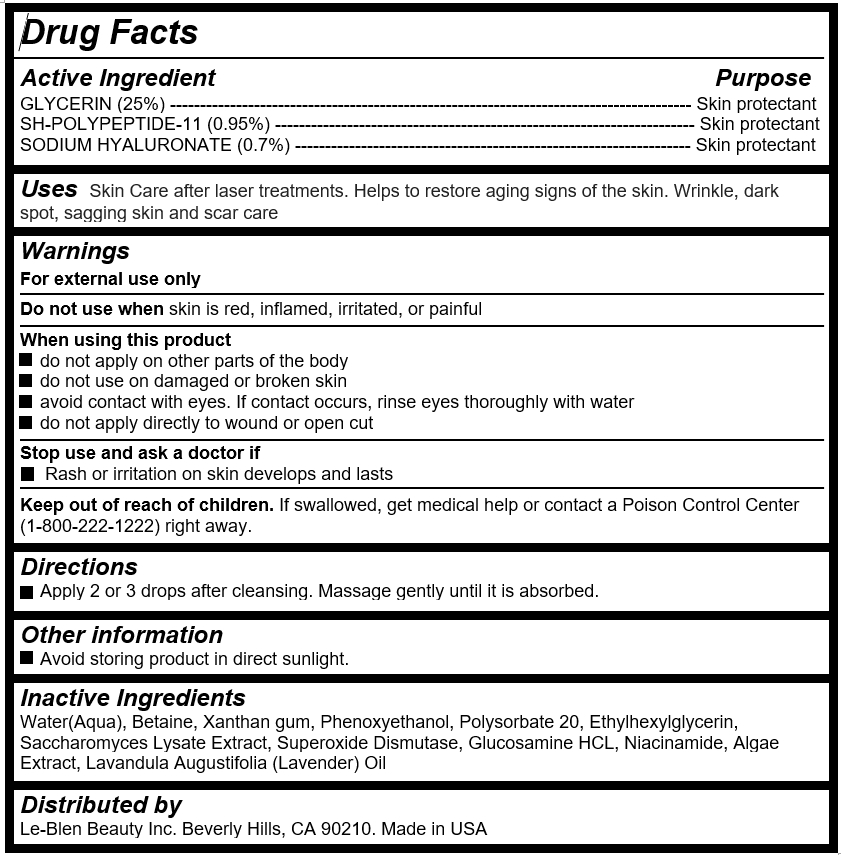
Active Ingredient
GLYCERIN (25%) --------------------------------------------------------------------------------------- Skin protectant
SH-POLYPEPTIDE-11 (0.95%) ---------------------------------------------------------------------- Skin protectant
SODIUM HYALURONATE (0.7%) ------------------------------------------------------------------ Skin protectant
Uses
Skin Care after laser treatments. Helps to restore aging signs of the skin. Wrinkle, dark spot, sagging skin and scar care
Warnings
For external use only
Do not use when skin is red, inflamed, irritated, or painful
When using this product
do not apply on other parts of the body
do not use on damaged or broken skin
avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
do not apply directly to wound or open cut
Stop use and ask a doctor if
Rash or irritation on skin develops and lasts
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.