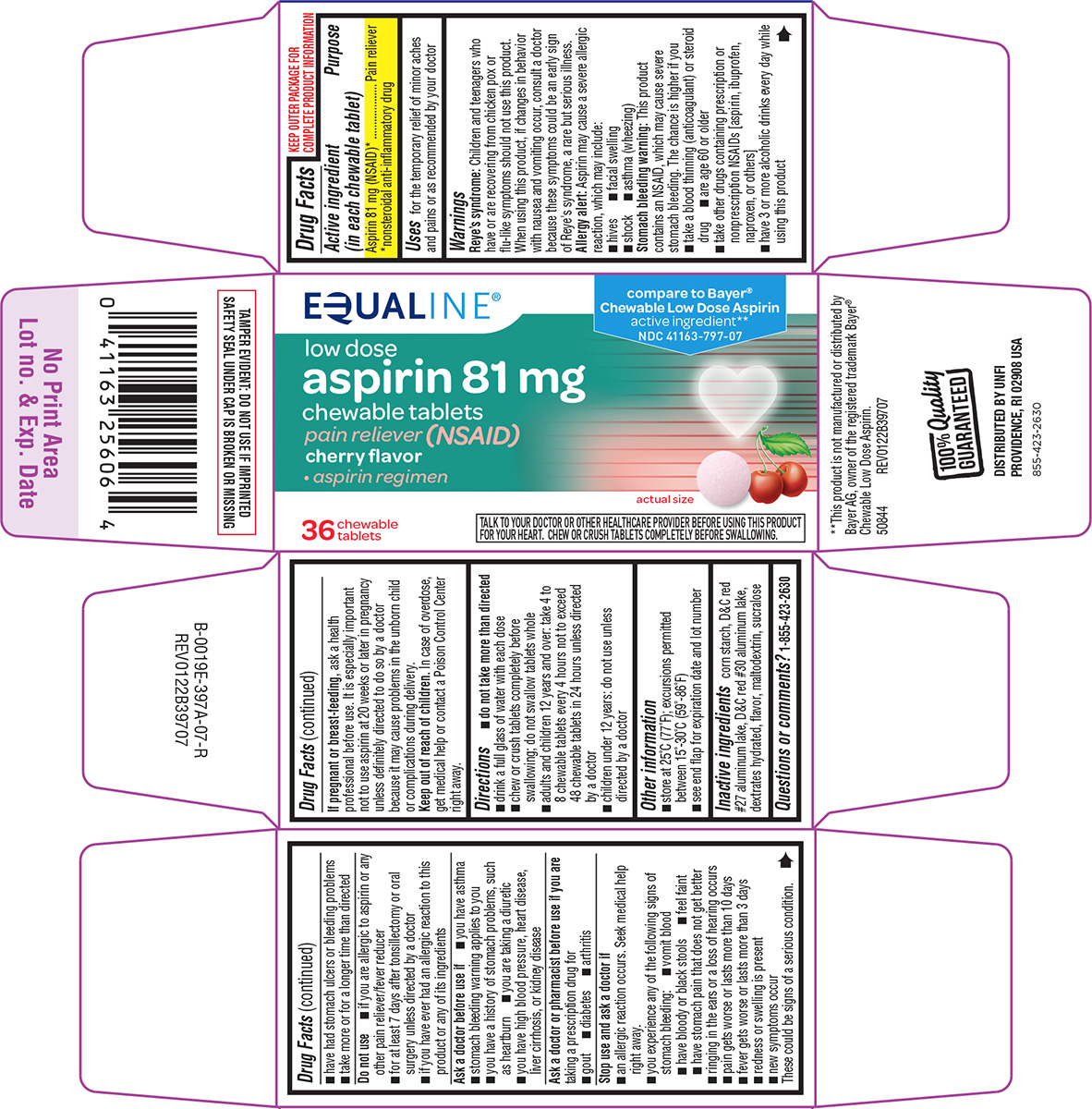
Active ingredient (in each chewable tablet)
Aspirin 81 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- hives
- facial swelling
- shock
- asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- take a blood thinning (anticoagulant) or steroid drug
- are age 60 or older
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- have had stomach ulcers or bleeding problems
- take more or for a longer time than directed
Do not use
- if you are allergic to aspirin or any other pain reliever/fever reducer
- for at least 7 days after tonsillectomy or oral surgery unless directed by a doctor
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you have asthma
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
taking a prescription drug for
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- vomit blood
- have bloody or black stools
- feel faint
- have stomach pain that does not get better
- ringing in the ears or a loss of hearing occurs
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
Directions
- drink a full glass of water with each dose
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- adults and children 12 years and over: take 4 to 8 chewable tablets every 4 hours not to exceed 48 chewable tablets in 24 hours unless directed by a doctor
- children under 12 years: do not use unless directed by a doctor
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrates hydrated, flavor, maltodextrin, sucralose
Principal display panel
Equaline®
compare to Bayer®
Chewable Low Dose Aspirin
active ingredient**
NDC 41163-797-07
low dose
aspirin 81 mg
chewable tablets
pain reliever (NSAID)
cherry flavor
• aspirin regimen
36 tablets
actual size
TALK TO YOUR DOCTOR OR OTHER HEALTHCARE PROVIDER BEFORE USING THIS PRODUCT
FOR YOUR HEART. CHEW OR CRUSH TABLETS COMPLETELY BEFORE SWALLOWING.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
**This product is not manufactured or distributed by
Bayer AG, owner of the registered trademark Bayer®
chewable Low Dose Aspirin.
50844 REV0122B39707
100% Quality
GUARANTEED
DISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USA
855-423-2630
Equaline 44-397A