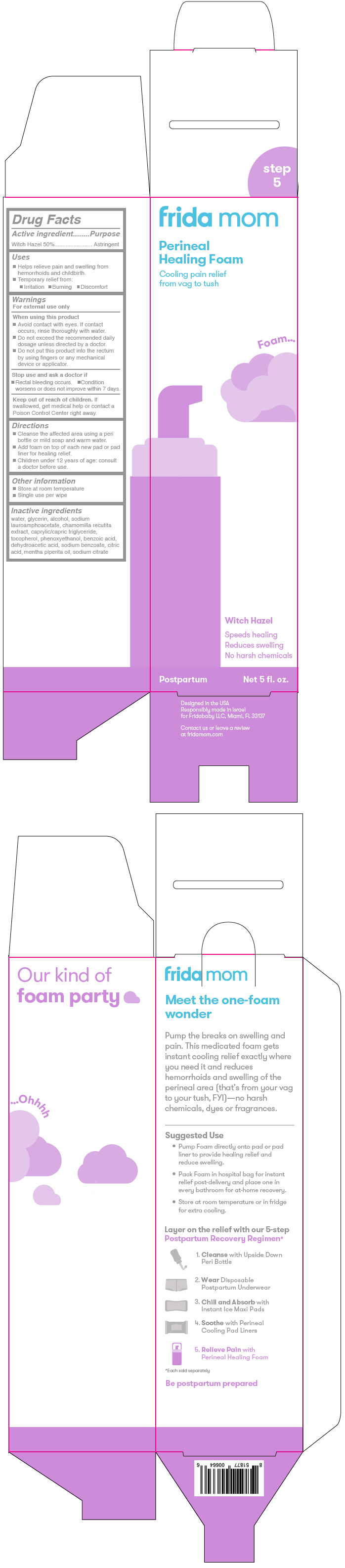
FRIDA MOM PERINEAL HEALING- witch hazel aerosol, foam
Fridababy, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Witch Hazel 50%
Uses
- Helps relieve pain and swelling from hemorrhoids and childbirth.
- Temporary relief from:
- Irritation
- Burning
- Discomfort
Warnings
For external use only
When using this product
- Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
- Do not exceed the recommended daily dosage unless directed by a doctor.
- Do not put this product into the rectum by using fingers or any mechanical device or applicator.
Stop use and ask a doctor if
- Rectal bleeding occurs.
- Condition worsens or does not improve within 7 days.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Cleanse the affected area using a peri bottle or mild soap and warm water.
- Add foam on top of each new pad or pad liner for healing relief.
- Children under 12 years of age: consult a doctor before use.
Other information
- Store at room temperature
- Single use per wipe
Inactive ingredients
water, glycerin, alcohol, sodium lauroamphoacetate, chamomilla recutita extract, caprylic/capric triglyceride, tocopherol, phenoxyethanol, benzoic acid, dehydroacetic acid, sodium benzoate, citric acid, mentha piperita oil, sodium citrate
PRINCIPAL DISPLAY PANEL - 5 fl. oz. Bottle Carton
frida mom
Perineal
Healing Foam
Cooling pain relief
from vag to tush
Foam...
Witch Hazel
Speeds healing
Reduces swelling
No harsh chemicals
Postpartum
Net 5 fl. oz.