FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ZELNORM is indicated for the treatment of adult women less than 65 years of age with irritable bowel syndrome with constipation (IBS-C).
Limitations of Use
- The safety and effectiveness of ZELNORM in men with IBS-C have not been established [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
The recommended dosage of ZELNORM in adult women less than 65 years of age is 6 mg taken twice daily orally at least 30 minutes before meals [see Clinical Pharmacology (12.3)]. Discontinue ZELNORM in patients who have not had adequate control of symptoms after 4 to 6 weeks of treatment.
3 DOSAGE FORMS AND STRENGTHS
ZELNORM Tablets: 6 mg tegaserod; supplied as whitish to slightly yellowish, round flat tablet with a beveled edge engraved with “ZEL” and “6”.
4 CONTRAINDICATIONS
ZELNORM is contraindicated in patients with:
- A history of myocardial infarction (MI), stroke, transient ischemic attack (TIA), or angina [see Warnings and Precautions (5.1)]
- A history of ischemic colitis or other forms of intestinal ischemia [see Warnings and Precautions (5.2)]
- Severe renal impairment (eGFR< 15 mL/min/1.73 m2) or end-stage renal disease [see Use in Specific Populations (8.6)]
- Moderate and severe hepatic impairment (Child-Pugh B or C) [see Use in Specific Populations (8.7)]
- A history of bowel obstruction, symptomatic gallbladder disease, suspected sphincter of Oddi dysfunction, or abdominal adhesions [see Adverse Reactions (6.2)]
- Hypersensitivity to tegaserod [see Adverse Reactions (6.2)]
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Ischemic Events, Including Major Adverse Cardiovascular Events (MACE)
Stroke, MI, and cardiovascular death (major adverse cardiovascular events [MACE]) have been reported in adults taking ZELNORM who had an increased risk of developing an adverse cardiovascular event based on their medical history [see Adverse Reactions (6.1)].
ZELNORM is contraindicated in patients with a history of MI, stroke, TIA, or angina [see Contraindications (4)]. Assess female patients less than 65 years of age for a history of cardiovascular disease and cardiovascular risk factors prior to treatment with ZELNORM [see Adverse Reactions (6.1)]. The potential risks of treatment must be balanced with expectations in improvements in symptoms of IBS-C.
Discontinue ZELNORM in patients who experience an MI, stroke, TIA, or angina [see Contraindications (4)]. Evaluate the risks and benefits of continued use of ZELNORM in patients who develop clinical or other evidence of cardiovascular ischemic heart disease (e.g., coronary artery disease) and/or experience changes in health status that could increase cardiovascular risk during treatment with ZELNORM.
5.2 Ischemic Colitis
Ischemic colitis and other forms of intestinal ischemia have been reported postmarketing in patients receiving ZELNORM [see Adverse Reactions (6.2)]. In some cases, hospitalization was required. Discontinue ZELNORM in patients who develop symptoms of ischemic colitis, such as rectal bleeding, bloody diarrhea, or new or worsening abdominal pain. Evaluate patients experiencing these symptoms promptly and perform appropriate diagnostic testing. Do not reinitiate ZELNORM in patients who develop findings consistent with ischemic colitis or other forms of intestinal ischemia [see Contraindications (4)].
5.3 Volume Depletion Associated with Diarrhea
Diarrhea is one of the most common adverse reactions in ZELNORM-treated patients from the pooled IBS-C double-blind, placebo-controlled trials. Diarrhea resulted in discontinuation in 1.6% of ZELNORM-treated patients compared to 0% in placebo [see Adverse Reactions (6)].
In postmarketing experience, serious consequences of diarrhea including hypovolemia, hypotension, and syncope have been reported in patients treated with ZELNORM. In some cases, these complications have required hospitalization for rehydration. Avoid use of ZELNORM in patients who are currently experiencing or frequently experience diarrhea. Instruct patients to discontinue ZELNORM and contact their healthcare provider if severe diarrhea, hypotension, or syncope occur.
5.4 Suicidal Ideation and Behavior
Suicide, suicidal attempt and ideation, and self-injurious behavior have been reported in clinical trials of IBS-C and other gastrointestinal motility disorders.The frequency of suicidal ideation or attempts with tegaserod treatment (8 patients out of 10,003) was higher than placebo (1 patient out of 5,425) [see Adverse Reactions (6.1)]. Suicidal ideation/behavior in clinical trials was proportionately more frequent among patients receiving antidepressant medication.
Monitor all ZELNORM-treated patients for clinical worsening of depression and emergence of suicidal thoughts and behaviors, especially during the initial few months of treatment. Counsel family members and caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Instruct patients to immediately discontinue ZELNORM and contact their healthcare provider if their depression is persistently worse or they are experiencing emergent suicidal thoughts or behaviors.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail elsewhere in the labeling:
- Cardiovascular Ischemic Events, including MACE [see Warnings and Precautions (5.1)]
- Ischemic Colitis [see Warnings and Precautions (5.2)]
- Volume Depletion Associated with Diarrhea [see Warnings and Precautions (5.3)]
- Suicidal Ideation and Behavior [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Common Adverse Reactions
In three clinical trials 2,343 female patients less than 65 years of age with IBS-C received ZELNORM 6 mg twice daily or placebo. The majority of patients were Caucasian. Table 1 provides the incidence of common adverse reactions reported in >2% of IBS-C patients in the ZELNORM treatment group and at an incidence that was greater than in the placebo group.
| Adverse Reactions | ZELNORM 6 mg twice daily [N = 1,184] % | Placebo [N = 1,159] % |
| Headache | 14 | 10 |
| Abdominal Painb | 11 | 10 |
| Nausea | 8 | 7 |
| Diarrhea | 8 | 3 |
| Flatulence | 6 | 5 |
| Dyspepsia | 4 | 3 |
| Dizziness | 4 | 3 |
| a Reported in >2% of ZELNORM-treated patients and at an incidence greater than placebo b Includes abdominal pain, upper abdominal pain, lower abdominal pain, abdominal discomfort, abdominal tenderness, epigastric pain or discomfort |
||
Diarrhea
The majority (84%) of the ZELNORM patients reporting diarrhea had a single episode. In most cases, diarrhea occurred within the first week of treatment. Typically, diarrhea resolved with continued therapy. Diarrhea resulted in discontinuation in 1.6% of ZELNORM-treated patients compared to 0% in placebo [see Warnings and Precautions (5.3)].
Less Common Adverse Reactions
The following is a list of less common adverse reactions reported in ≤ 2% of patients in clinical trials of IBS-C on ZELNORM but more frequently than placebo:
Blood and Lymphatic System Disorders: Anemia
Ear and Labyrinth Disorders: Vertigo
Gastrointestinal Disorders: Rectal hemorrhage
General Disorders and Administration Site Conditions: Asthenia
Investigations: Increased blood creatine phosphokinase
Metabolism and Nutrition Disorders: Increased appetite
Musculoskeletal and Connective Tissue Disorders: Arthropathy, tendonitis
Nervous System Disorders: Migraine
Adverse Reactions of Special Interest
ZELNORM is recommended for use in female patients with IBS-C, and is not recommended for other motility disorders [see Indications and Dosage (1)].
Major Adverse Cardiovascular Events (MACE)
A retrospective analysis of the pooled clinical trial database data (involving 18,645 patients, both male and female) of 29 placebo-controlled trials of IBS-C and other gastrointestinal motility disorders of at least four weeks duration was conducted. An external adjudication of the reported cardiovascular ischemic (CVI) events identified an imbalance in patients taking ZELNORM (13 events, 0.1%) compared to placebo (1 event, 0.01%). A second external adjudication was conducted with additional patient-level information, and used a comprehensive pre-specified methodology regarding both case selection and assessment. This adjudication confirmed seven CVI events (0.06%) on ZELNORM compared to one event (0.01%) on placebo. An imbalance in MACE events (defined as cardiovascular death, non-fatal MI, non-fatal stroke) was observed in patients taking ZELNORM compared to placebo, as reported in both external adjudications. All events occurred in male and female patients with a history of cardiovascular ischemic disease and/or more than one cardiovascular risk factor.
A summary of the event rates from both adjudications is provided in Table 2. The rate of MACE events for ZELNORM-treated patients ranged from 0.03% to 0.06% in the overall population and 0.01% to 0.03% in the female population less than 65 years of age without a history of cardiovascular ischemic disease compared to zero in the placebo-treated group.
| All Patients (Male and Female) | Females < 65 Years of Age | |||||
| Without a History of Cardiovascular Ischemic Diseasea | Without a History of Cardiovascular Ischemic Diseasea
and One or Fewer Cardiovascular Risk Factorsb |
|||||
| ZELNORM (N=11,614) | Placebo (N=7,031) | ZELNORM (N=9,547) | Placebo (N=5,748) | ZELNORM (N=7,785) | Placebo (N=4,686) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| First External Adjudication | 7c | 0 | 3e | 0 | 0 | 0 |
| 0.06% | 0.03% | |||||
| Second External Adjudication | 4d | 0 | 1f | 0 | 0 | 0 |
| 0.03% | 0.01% | |||||
| a
Defined as prior MI, stroke, transient ischemic attack, angina, etc. b Defined as active smoking, current hypertension/history of antihypertensive treatment, current hyperlipidemia/history of lipid lowering medication, history of diabetes mellitus, age ≥55 years, or obesity (BMI >30 kg/m2) c Five females less than 65 years, one male less than 65 years and one male greater than 65 years of age d Three females less than 65 years of age and one male greater than 65 years of age e Cardiovascular death, MI and stroke; all three patients had > one cardiovascular risk factor at baseline f Cardiovascular death (one of the three cases confirmed in the 1st external adjudication) |
||||||
Suicidal Ideation/Behavior
Two ZELNORM-treated patients committed suicide, one in a controlled study of IBS-C and one during open label treatment for another motility disorder. In 27 placebo-controlled trials, assessing tegaserod at a total daily dose of 4 mg to 50 mg (up to four times the recommended daily dose), or placebo for the treatment of IBS-C or other gastrointestinal motility disorders, the frequency of suicidal ideation/behavior with tegaserod treatment (8 events/10,003, or 0.08%) was higher than placebo (1 event/5,425, or 0.02%). Events on ZELNORM included one completed suicide, two suicide attempts, four cases of self-injurious behavior, and one case of suicidal ideation. There was one suicide attempt on placebo. Of the eight ZELNORM-treated patients who experienced an event, all were less than 65 years of age, seven were female and three had IBS-C. The patient who committed suicide was a female, less than 65 years of age with IBS-C, taking ZELNORM 2 mg twice daily.
Abdominal Surgeries, Including Cholecystectomy
An increase in abdominal surgeries was observed on ZELNORM (9 patients out of 2,965 or 0.3%) versus placebo (3 patients out of 1,740 or 0.2%) in clinical trials of men and women treated with ZELNORM for IBS-C. The increase was primarily due to a numerical imbalance in cholecystectomies reported in patients treated with ZELNORM (5 patients out of 2,965 or 0.17%) versus placebo (1 patient out of 1,740 or 0.06%). A causal relationship between abdominal surgeries and ZELNORM has not been established.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ZELNORM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Ischemic colitis, mesenteric ischemia, gangrenous bowel and rectal bleeding [see Warnings and Precautions (5.2)]
- Severe diarrhea resulting in syncope, hypotension, hypovolemia, electrolyte disorders [see Warnings and Precautions (5.3)]
- Sphincter of Oddi spasm, bile duct stone, cholecystitis with elevated transaminases, elevation in ALT, AST and bilirubin, hepatitis [see Contraindications (4)]
- Alopecia
- Hypersensitivity reactions, including anaphylaxis [see Contraindications (4)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from case reports with ZELNORM use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, decreased survival of rat pups was observed with maternal dietary administration of tegaserod at 71 times the recommended dose during organogenesis and through lactation. Decreased body weight and delays in developmental landmarks in rat pups were observed with maternal dietary administration of 45 times the recommended dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
No adverse developmental effects were observed with oral administration of tegaserod to pregnant rats at doses up to 100 mg/kg/day (approximately 15 times the recommended dose based on area under the plasma concentration-time curve [AUC]) or to pregnant rabbits at doses up to 120 mg/kg/day (approximately 51 times the recommended dose based on AUC) during organogenesis.
A pre- and postnatal developmental toxicity study was performed in rats using dietary administration of up to 300 mg/kg/day (71 times the recommended dose based on AUC) during organogenesis and through lactation. The survival rate through postnatal days 4 and 21 was 59% at 300 mg/kg/day as compared to 95% to 99% in the control group. At doses of 150 mg/kg/day and higher (45 times the recommended dose based on AUC), decreased body weight and delays in developmental landmarks were observed. No adverse effects were observed at 75 mg/kg/day (14 times the recommended dose based on AUC).
8.2 Lactation
Risk Summary
There are no data regarding the presence of tegaserod in human milk, the effects on the breastfed infant, or the effects on milk production. Tegaserod and its metabolites are present in rat milk; the milk to plasma concentration ratio for tegaserod is very high (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the potential for serious reactions in the breastfed infant, including tumorigenicity [see Nonclinical Toxicology (13.1)], advise a lactating woman that breastfeeding is not recommended during treatment with ZELNORM.
Data
Following oral administration, tegaserod and its metabolites are excreted in the milk of lactating rats with a milk to plasma concentration ratio of 33:1 at eight hours.
8.4 Pediatric Use
Safety and effectiveness of ZELNORM in pediatric patients have not been established.
8.6 Renal Impairment
ZELNORM is contraindicated in patients with severe renal impairment (eGFR < 15 mL/min/1.73 m2) or end stage renal disease [see Contraindications (4)]. The Cmax and AUC of the tegaserod metabolite, 5-methoxyindole-3-carboxylic acid glucuronide (M29), are substantially increased in severe renal impairment [see Clinical Pharmacology (12.3)].
No dosage adjustment is recommended in patients with mild to moderate renal impairment (eGFR ≥ 30 mL/min/1.73 m2).
10 OVERDOSAGE
Single oral doses of 120 mg (20 times the recommended dose) of ZELNORM were administered to three healthy subjects in one study. All three subjects developed diarrhea and headache. Two of these subjects also reported intermittent abdominal pain and one developed orthostatic hypotension. In 28 healthy subjects exposed to 90 to 180 mg per day of ZELNORM (7.5 to 15 times the recommended daily dosage) for several days, adverse reactions were diarrhea (100%), headache (57%), abdominal pain (18%), flatulence (18%), nausea (7%), and vomiting (7%).
Based on the large distribution volume and high protein binding of tegaserod, it is unlikely that tegaserod could be removed by dialysis. In cases of overdosage, treat symptomatically and institute supportive measures as appropriate.
11 DESCRIPTION
ZELNORM oral tablets contain tegaserod, a serotonin-4 (5-HT4) receptor agonist, as the hydrogen maleate salt. As the maleate salt, tegaserod is chemically designated as 3-(5-methoxy-1H-indol-3-ylmethylene)-N-pentylcarbazimidamide hydrogen maleate. Its empirical formula is C16H23N5O•C4H4O4. The molecular weight is 417.47 and the structural formula is:
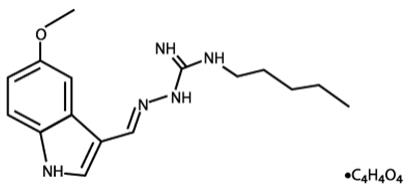
Tegaserod as the maleate salt is a white to off-white crystalline powder and is slightly soluble in ethanol and very slightly soluble in water. Each ZELNORM tablet contains 6 mg of tegaserod (equivalent to 8.31 mg of tegaserod maleate) and the following inactive ingredients: colloidal silicon dioxide, crospovidone, glyceryl behenate, hypromellose, and lactose monohydrate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tegaserod is an agonist of serotonin type-4 (5-HT4) receptors that stimulates the peristaltic reflex and intestinal secretion, inhibits visceral sensitivity, enhances basal motor activity, and normalizes impaired motility throughout the gastrointestinal tract.
Based on in vitro binding affinity and functional assessment, at clinically relevant plasma concentrations, tegaserod is an antagonist at 5-HT2B receptors in humans. It is expected to have minimal binding to 5-HT1 receptors. Tegaserod has no affinity for 5-HT3 or dopamine receptors.
The main metabolite, M29, has negligible affinity for 5-HT4 receptors in vitro.
In vivo studies showed that tegaserod enhanced basal motor activity and normalized impaired motility throughout the gastrointestinal tract. In addition, studies demonstrated that tegaserod moderated visceral sensitivity during colorectal distension in animals.
12.2 Pharmacodynamics
Cardiac Electrophysiology
Centrally analyzed ECGs were recorded in 4,605 male and female patients receiving ZELNORM 6 mg twice daily or placebo for IBS-C and other related motility disorders. No subject receiving tegaserod had an absolute QTcF above 480 ms. An increase in QTcF of 30 to 60 ms was observed in 7% of patients receiving ZELNORM and 8% receiving placebo. An increase in QTcF of greater than 60 ms was observed in 0.3% and 0.2% of subjects, respectively. The effects of tegaserod on the QTcF interval were not considered to be clinically meaningful.
Platelet Aggregation
There is a potential for tegaserod and its main metabolite (M29) to increase platelet aggregation in vitro. In one in vitro study, tegaserod, at concentrations up to 10 times the maximum plasma concentration (Cmax) at the recommended dose, significantly increased platelet aggregation in a concentration-dependent manner up to 74% (range 11% to 74%) compared to vehicle control (with potentiation by various agonists). In another in vitro study, M29, at concentrations up to 0.6 times the Cmax of M29 also showed a 5% to 16% increase in platelet aggregation compared to vehicle control. The clinical implications of the in vitro platelet aggregation results are unclear.
12.3 Pharmacokinetics
The pharmacokinetics of tegaserod in IBS-C patients are comparable to those in healthy subjects. The mean (±SD) peak tegaserod concentration (Cmax) was 2.9 (±1.1) ng/mL, and mean (±SD) AUC was 10.5 (±4.6) h•ng/mL following a single ZELNORM dose at 6 mg. Tegaserod systemic exposure at steady state increase proportionally over a dose range of 2 mg to 12 mg twice daily (0.3 to 2 times the approved recommended dosage). There was no significant accumulation (~10%) of tegaserod following the approved recommended dosage.
Absorption
The absolute bioavailability of tegaserod is approximately 10% when administered to fasting subjects. The median time to peak tegaserod plasma concentration (Tmax) is approximately one hour (range 0.7 to 2 hours).
Effect of Food
Compared to under fasted conditions, the tegaserod AUC was reduced by 40% to 65%, Cmax was reduced by approximately 20% to 40% and median Tmax was 0.7 hours when ZELNORM was administered 30 minutes before a high-fat, high-calorie meal (approximately 150 calories from protein, 250 calories from carbohydrates, and 500 calories from fat). Plasma concentrations were similar when ZELNORM was administered within 30 minutes prior to a meal or 2.5 hours after a meal [see Dosage and Administration (2)].
Distribution
Protein binding of tegaserod is approximately 98%. The mean volume of distribution of tegaserod (± SD) at steady-state is 368 ± 223 L following intravenous administration (ZELNORM is not approved for intravenous administration).
Elimination
The mean tegaserod terminal elimination half-life ranged from 4.6 to 8.1 hours following oral administration and the mean (± SD) plasma clearance was 77 ± 15 L/h following intravenous administration.
Metabolism
Tegaserod is metabolized via hydrolysis and direct glucuronidation. Tegaserod undergoes hydrolysis in the stomach followed by oxidation and conjugation which produces the M29 metabolite.
Excretion
Approximately two-thirds of a ZELNORM dose is excreted unchanged in the feces, with the remaining one-third excreted in the urine as metabolites.
Specific Populations
Patients with Renal Impairment
No change in the pharmacokinetics of tegaserod was observed in subjects with end stage renal disease (creatinine clearance normalized by body surface area (CrCL) < 15 mL/min/1.73 m2) requiring hemodialysis. Although renal impairment does not affect the pharmacokinetics of tegaserod, the pharmacokinetics of its main metabolite (M29) are altered, the Cmax of M29 doubling and the AUC increasing 10-fold in patients with severe renal impairment (CrCL < 15 mL/min/1.73 m2) compared to healthy subjects with normal renal function (CrCL > 80 mL/min/1.73 m2) [see Contraindications (4), Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
In subjects with mild hepatic impairment (Child-Pugh A), the mean tegaserod AUC was 31% higher and the Cmax was 16% higher compared to healthy subjects with normal hepatic function. The increase in exposure in subjects with mild impairment is not considered to be clinically relevant.
In a single subject with moderate hepatic impairment, the Cmax and AUC were 140% and 200% of that observed in healthy controls. ZELNORM has not been studied in patients with moderate or severe hepatic impairment (Child-Pugh B or C) [see Contraindications (4), Use in Specific Populations (8.7)].
Drug Interaction Studies
Effect of Other Drugs on Tegaserod
Quinidine: Coadministration of a single dose of 600 mg quinidine (P-gp inhibitor) with a single dose of ZELNORM 6 mg increased the mean tegaserod AUC(0-12h) and the mean Cmax by 50% and 44%, respectively, compared to ZELNORM administered alone. Coadministration of multiple doses of quinidine (600 mg once daily for three days) with ZELNORM 6 mg twice daily for six days increased the mean tegaserod AUC(0-12h) and Cmax by 71% and 63%, respectively, compared to ZELNORM administered alone.
Inhibitors of P-gp (e.g., ritonavir, clarithromycin, itraconazole) may modestly increase the oral bioavailability of tegaserod. The clinical relevance of increased systemic exposure as a result of P-gp inhibition is unclear.
Omeprazole: Administration of omeprazole 20 mg once daily for four days followed by ZELNORM 6 mg twice daily on day four increased the mean tegaserod AUC and Cmax by 15% and 17%, respectively, compared to ZELNORM administered alone. This increase in exposure is not considered clinically relevant.
Effect of Tegaserod on Other Drugs
No clinically significant effects of tegaserod on the pharmacokinetics of the following drugs were observed when used concomitantly with a single dose of ZELNORM 6 mg: theophylline (CYP1A2 substrate), dextromethorphan (CYP2D6 substrate), digoxin (P-gp substrate), warfarin (CYP2C9 substrate), or oral contraceptives (ethynyl estradiol and levonorgestrel).
Digoxin: Administration of a single dose of digoxin following ZELNORM 6 mg twice daily for 4 days reduced the mean Cmax and AUC of digoxin by approximately 15%. This reduction in digoxin exposure is not considered clinically relevant.
Warfarin: Coadministration of ZELNORM 6 mg twice daily with warfarin for seven days did not significantly alter the pharmacokinetics of either R- or S-warfarin or change the prothrombin time in healthy subjects.
Oral Contraceptives: Coadministration of ZELNORM 6 mg twice daily with 0.3 mg of ethinyl estradiol and 0.125 mg of levonorgestrel once daily did not affect the steady-state (Day 21) pharmacokinetics of ethinyl estradiol but reduced both the Cmax and AUC of levonorgestrel by 8%. This change in exposure is not considered clinically relevant.
In Vitro Studies Where the Drug Interaction Potential Was Not Further Evaluated Clinically
CYP enzymes
Tegaserod does not inhibit CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, and CYP3A4, and it does not induce CYP3A4 and CYP2B6.
Limited induction of CYP1A2 was observed at tegaserod concentrations in excess of 100 times the clinically relevant range.
M29 does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4, and it does not induce CYP1A2, CYP2B6, or CYP3A4.
Transporters
Tegaserod is a substrate for BCRP and P-gp, but not a substrate of OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, MATE1, MATE2-K or BSEP. Drug transporter data indicated a potential inhibition of MATE1, MATE2-K, and BCRP by tegaserod at high concentrations. However, at the clinical dose of ZELNORM, a significant in vivo drug interaction via inhibition of these transporters is unlikely.
M29 is a substrate of BCRP, P-gp, OAT3 and BSEP transporters, but not a substrate of OAT1, OCT1, OCT2, OATP1B1, OATP1B3, MATE1, and MATE2-K. M29 does not inhibit the following transporters: OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, MATE1, MATE2-K, BCRP, P-gp, and BSEP.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Tegaserod was not carcinogenic in rats given oral dietary doses up to 180 mg/kg/day (approximately 93 to 111 times the recommended dose based on AUC) for 110 to 124 weeks.
In mice, dietary administration of tegaserod for 104 weeks produced mucosal hyperplasia and adenocarcinoma of the small intestine at 600 mg/kg/day (approximately 83 to 110 times the recommended dose based on AUC). There was no evidence of carcinogenicity at lower doses (3 to 35 times the recommended dose based on AUC).
Tegaserod was not genotoxic in the in vitro Chinese hamster lung fibroblast (CHL/V79) cell chromosomal aberration and forward mutation test, the in vitro rat hepatocyte unscheduled DNA synthesis (UDS) test or the in vivo mouse micronucleus test. The results of the Ames test for mutagenicity were equivocal.
Tegaserod at oral (dietary) doses up to 240 mg/kg/day (approximately 57 times the recommended dose based on AUC) in male rats and 150 mg/kg/day (approximately 42 times the recommended dose based on AUC) in female rats was found to have no effect on fertility and reproductive performance.
13.2 Animal Toxicology and/or Pharmacology
Inhibition of the hERG (human Ether-a-go-go-Related Gene) channel was evident only in the micromolar concentration range with an IC50 of 13 micromolar (approximately 1300 times the Cmax in humans at the recommended dose). In in vitro studies, tegaserod had no effects on impulse conduction in isolated guinea pig papillary muscle at up to 100 times the Cmax in humans, Langendorff-perfused isolated rabbit heart (QT interval) at up to 1000 times the Cmax in humans, or human atrial myocytes at multiples up to 10 times the Cmax in humans. The major metabolite, M29, had no effect on QT in the Langendorff-perfused isolated rabbit heart at multiples up to 323 times the Cmax in humans.
In anesthetized and conscious dogs, tegaserod at doses up to 92 to 134 times the recommended dose based on Cmax did not alter heart rate, QRS interval duration, QTc or other ECG parameters. In chronic toxicology studies in rats and dogs, there were no treatment-related changes in cardiac morphology after tegaserod administration at doses up to 660 times the recommended dose based on AUC.
Although tegaserod is expected to bind to 5 HT2B receptors in humans at the recommended dose, there does not appear to be any potential for heart valve injury based on functional evidence of 5 HT2B receptor antagonism.
Studies with isolated coronary and mesenteric blood vessels from non-human primates and humans showed no vasoconstrictor effect at concentrations approximately 100 times the human Cmax. Tegaserod exhibited antagonism of 5 HT-mediated vasoconstriction via 5 HT1B receptors. In rat thoracic aortic rings that were pre-constricted with phenylephrine or norepinephrine, tegaserod produced vasorelaxation, with IC50 values 6 and 64 times the Cmax plasma concentrations in humans, respectively. No effects were observed in the basal tone of aortic rings at concentrations up to 1000 times the human Cmax.
In studies with an anesthetized rat model for measuring macro- and micro-circulation of the colon, intraduodenal dosing with tegaserod (approximately 7 times the recommended dose based on Cmax) produced no clinically relevant effect on blood pressure, heart rate, or vascular conductance.
14 CLINICAL STUDIES
Results in Women
ZELNORM is not recommended in females 65 years of age and older with IBS-C [see Indications and Dosage (1)].
In three multicenter, double-blind, placebo-controlled trials, 2,470 women (mean age 43 years [range 17 to 89 years]; 86% Caucasian, 10% African American) with at least a 3-month history of IBS-C symptoms prior to the baseline period that included abdominal pain, bloating and constipation received either ZELNORM 6 mg twice daily or placebo. In all patients, constipation was characterized by at least two of the following three symptoms each occurring >25% of the time over a 3-month period: <3 bowel movements/week, hard or lumpy stools, or straining with a bowel movement.
The design for the three trials consisted of a 4-week placebo-free baseline period followed by a 12-week double-blind treatment period. Studies 1 and 2 evaluated a fixed dose regimen of tegaserod 6 mg twice daily while Study 3 utilized a dose-titration design.
Each week of the 4-week placebo-free baseline period and the 12-week double-blind treatment period, patients were asked the question, “Please consider how you felt this past week in regard to your IBS, in particular your overall well-being, and symptoms of abdominal discomfort, pain and altered bowel habit. Compared to the way you usually felt before entering the trial, how would you rate your relief of symptoms during the past week?” The response variable consisted of the following five categories: completely relieved, considerably relieved, somewhat relieved, unchanged, or worse. Patients were classified as responders within a month if they were considerably or completely relieved for at least two of the four weeks, or if they were at least somewhat relieved for each of the four weeks.
Calculated response rates during month 1 and during month 3, as described above, are shown in Table 3. The differences in response rates vs. placebo were greater at month 1 than month 3.
| Study | Month 1 | Month 3** | ||||
| Proportion of Responders (Female) | Proportion of Responders (Female) | |||||
| ZELNORM
6 mg twice daily | Placebo | Difference
[95% CI] | ZELNORM
6 mg twice daily | Placebo | Difference
[95% CI] |
|
| 1 | 76/244 | 42/240 | 14% | 95/244 | 66/240 | 11% |
| (31%) | (17%) | [6%,21%] | (39%) | (28%) | [3%,20%] | |
| 2 | 265/767 | 164/752 | 13% | 334/767 | 292/752 | 5% |
| (35%) | (22%) | [8%,17%] | (44%) | (39%) | [0%,10%] | |
| 3 | 80/233 | 47/234 | 14% | 100/233 | 88/234 | 5% |
| (34%) | (20%) | [6%,22%] | (43%) | (38%) | [-4%,14%] | |
| *A responder is defined as a patient with ≥ 2 of 4 weeks with complete or considerable relief or 4 of 4 weeks with at least somewhat relief during the last 4 available weeks. **Primary efficacy assessment. |
||||||
In a subgroup of female patients less than 65 years of age (90%, 97%, and 91% of all female patients in Studies 1, 2 and 3, respectively), the treatment differences were generally similar at both month 1 and month 3 to the overall results shown in Table 3.
The same efficacy variable (i.e., complete relief, considerable relief, somewhat relief, unchanged, worse) was analyzed on a weekly basis. The proportion of all female patients with complete, considerable or somewhat relief at weeks 1, 4, 6, 8 and 12 are shown in Figure 1 below.
Figure 1: Weekly Proportion of Patients with Somewhat, Considerably and Complete Relief in the Three Placebo-Controlled IBS-C Trials
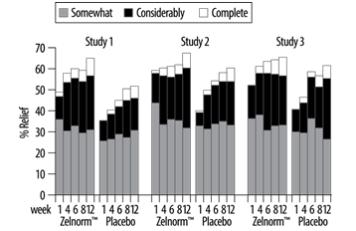
In addition, individual symptoms of abdominal pain/discomfort and bloating were assessed daily using a six or seven point intensity scale. A positive response was defined as at least a 1-point reduction in the scale. During the first four weeks in the fixed dose trials, 8% to 11% more ZELNORM-treated patients than placebo-treated patients were responders for abdominal pain/discomfort. Similarly, 9% to 12% more ZELNORM-treated patients were responders for bloating. Corresponding differences at month 3 were 1% to 10% responders for abdominal pain/discomfort and 4% to 11% responders for bloating. Patients on ZELNORM also experienced an increase in median number of bowel movements from 3.8/week at baseline to 6.3/week at month 1 and 6.0/week at month 3, while placebo patients increased from 4.0/week to 5.1/week at month 1 and 5.5/week at month 3.
Results in Men
In two randomized, placebo-controlled, double-blind trials enrolling 288 males, efficacy response rates were similar between ZELNORM and placebo in the male subgroup [see Indications and Usage (1)].
16 HOW SUPPLIED / STORAGE AND HANDLING
ZELNORM is supplied as 6 mg tegaserod whitish to slightly yellowish, round flat tablets with a beveled edge engraved with “ZEL” and “6”.
Unit Dose (blister pack)
- Box of 60 (strips of 10) NDC 0525-0971-60
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
See USP Controlled Room Temperature. Protect from moisture.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Cardiovascular Ischemic Events, Including MACE
Inform patients that stroke, myocardial infarction, and cardiovascular death have been reported in adults taking ZELNORM who had an increased risk of developing an adverse cardiovascular event based on their medical history. Advise patients to promptly seek medical attention if they develop symptoms of an MI, stroke, TIA, or angina. Also, advise patients to immediately inform their healthcare provider if they develop clinical or other evidence of cardiovascular ischemic heart disease (e.g., coronary artery disease) or other changes in their health status that could increase cardiovascular risk (e.g., smoking, hypertension, hyperlipidemia, diabetes mellitus, obesity) while taking ZELNORM [see Warnings and Precautions (5.1)].
Ischemic Colitis
Advise patients to stop taking ZELNORM and seek medical attention if they develop symptoms of ischemic colitis, such as rectal bleeding, bloody diarrhea or new or worsening abdominal pain [see Warnings and Precautions (5.2)].
Volume Depletion Associated with Diarrhea
Instruct patients to discontinue ZELNORM and contact their healthcare provider if they develop severe diarrhea, especially if they also experience symptoms of volume depletion (hypotension and/or syncope) [see Warnings and Precautions (5.3)].
Suicidal Ideation and Behavior
Counsel patients, their caregivers, and their families that ZELNORM may increase the risk of suicidal thoughts and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts of self-harm. Instruct patients, caregivers, and families that if any of these symptoms occur, they should immediately discontinue ZELNORM and report behaviors of concern to their healthcare provider [see Warnings and Precautions (5.4)].
Lactation
Advise a woman that breastfeeding is not recommended during treatment with ZELNORM [see Use in Specific Populations (8.2)].
Administration Information
Advise patients to take ZELNORM at least 30 minutes before a meal [see Dosage and Administration (2)].
Made in Germany
Distributed by:
Alfasigma USA, Inc.
Covington, LA 70433 USA
©2019. ZELNORM is a trademark of Alfasigma, USA Inc. All rights reserved.
PM-000413
| Medication Guide
ZELNORMª (ZEL-norm) (tegaserod) tablets, for oral use |
| What is the most important information I should know about ZELNORM?
ZELNORM can cause serious side effects, including:
Get emergency medical help right away if you have signs or symptoms of a heart attack, stroke, mini stroke (transient ischemic attack or TIA) or angina (chest pain that happens because your heart is not getting enough oxygen), including: • chest pain that may spread to the arms, neck, jaw, back, or stomach area (abdomen) • feeling sweaty • shortness of breath • feeling sick or vomiting • sudden numbness or weakness, especially on one side of the body • severe headache or confusion • problems with vision, speech, or balance Tell your healthcare provider right away if you are told that you have narrowing in the blood vessels that carry blood to your heart (coronary artery disease).
|
| What is ZELNORM?
ZELNORM is a prescription medicine used for the treatment of adult women less than 65 years of age who have irritable bowel syndrome with constipation (IBS-C). It is not known if ZELNORM is safe and effective in men with IBS-C. It is not known if ZELNORM is safe and effective in children. |
Do not take ZELNORM if you:
|
Before taking ZELNORM, tell your healthcare provider about all of your medical conditions, including if you:
|
How should I take ZELNORM?
|
| What are the possible side effects of ZELNORM?
ZELNORM can cause serious side effects, including:
• headache • stomach-area (abdominal) pain • nausea • gas • indigestion • dizziness These are not all the possible side effects of ZELNORM. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
How should I store ZELNORM?
|
| General information about the safe and effective use of ZELNORM.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZELNORM for a condition for which it was not prescribed. Do not give ZELNORM to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ZELNORM that is written for health professionals. |
| What are the ingredients in ZELNORM?
Active ingredient: tegaserod Inactive Ingredients: colloidal silicon dioxide, crospovidone, glyceryl behenate, hypromellose, and lactose monohydrate  Distributed by: Alfasigma USA, Inc., Covington, LA 70433 USA ©2019. ZELNORM is a trademark of Alfasigma, USA Inc. All rights reserved. For more information, call 1‑855‑697‑9232. |
| This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: July 2019 PM-000413 |
PRINCIPAL DISPLAY PANEL
NDC 0525-0971-30
Zelnorm
(tegaserod) tablets
6 mg per tablet
30 tablets (3 blister cards of 10 tablets each)