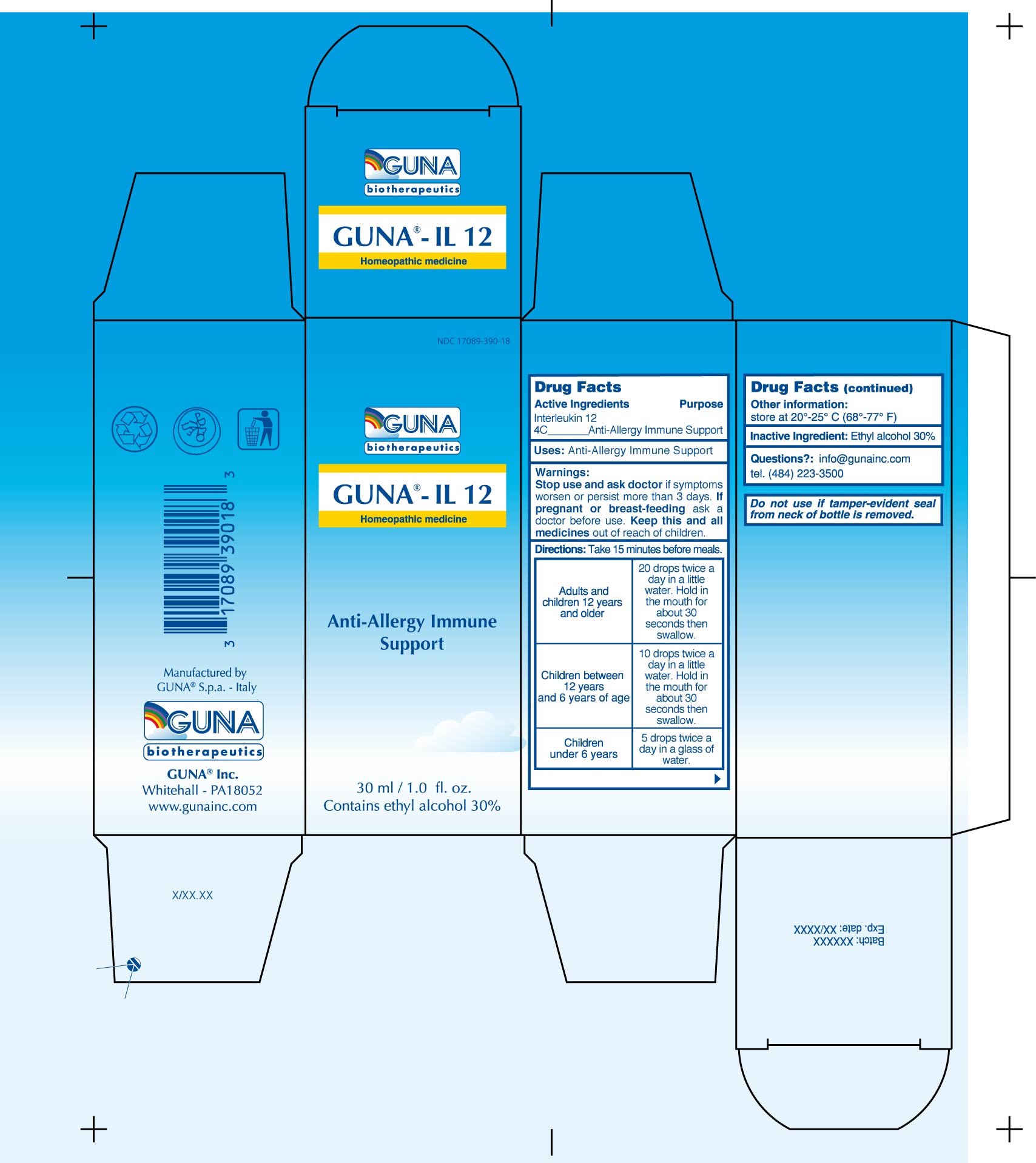
GUNA-IL 12- interleukin-12 human recombinant solution/ drops
Guna spa
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS/PURPOSE
INTERLEUKIN-12 4C ANTI-ALLERGY IMMUNE SUPPORT
USES
ANTI-ALLERGY IMMUNE SUPPORT
WARNINGS
Stop use and ask doctor if symptoms worsen or persist more than 3 days
PREGNANCY
If pregnant or breast-feeding ask a doctor before use
WARNINGS
Keep this and all medicines out of reach of children
DIRECTIONS
Take 15 minutes before meals.
Adults and children 12 years and older 20 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children between 12 years and 6 years of age 10 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children under 6 years 5 drops twice a day in a glass of water.
QUESTIONS
Questions?: info@gunainc.com
Tel. (484) 223-3500
Take 15 minutes before meals.
Inactive ingredient: Ethyl alcohol 30%.
PRINCIPAL DISPLAY PANEL