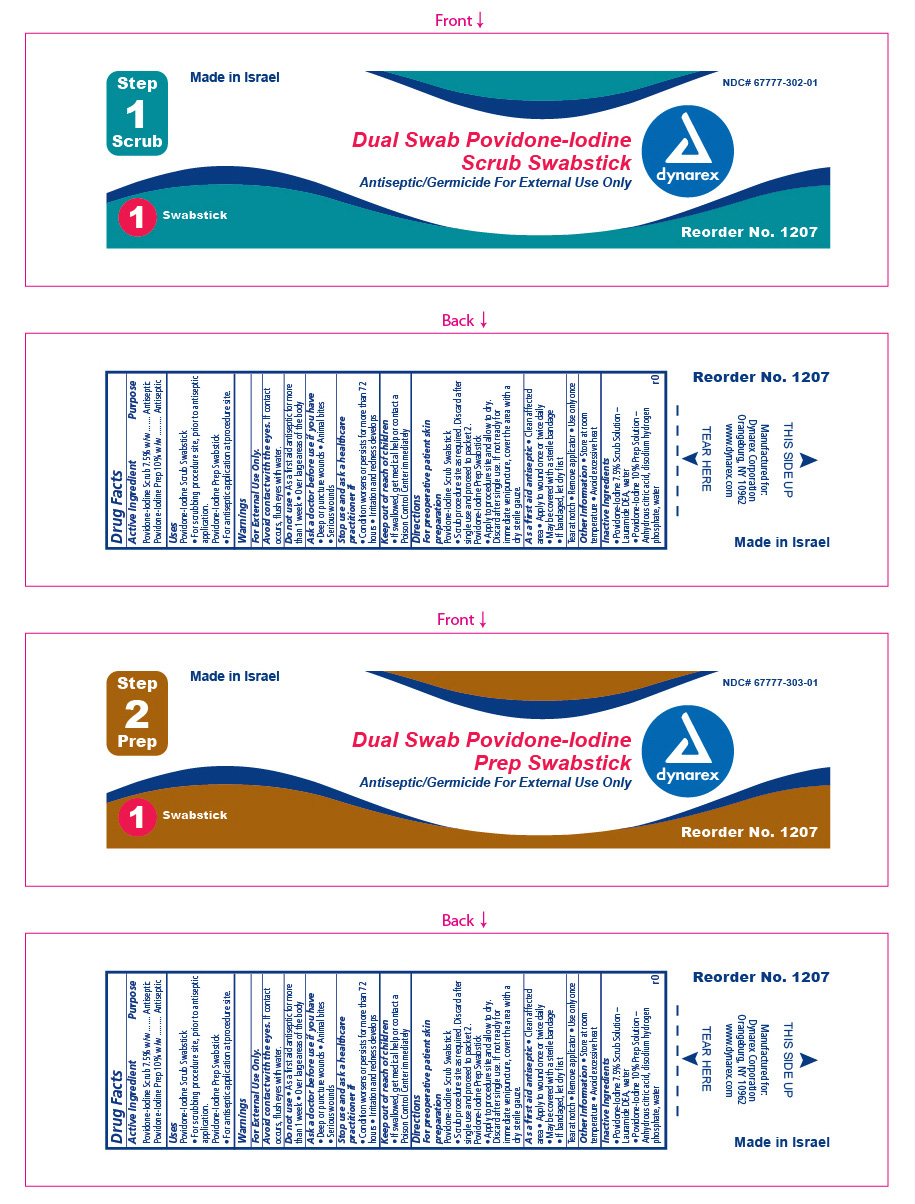
Active Ingredient Purpose
Povidone Iodine Scrub 7.5% w/w Antiseptic
Povidone Iodine Prep 10% w/w Antiseptic
Purpose
Uses
Povidone Iodine Scrub Swabstick - For scrubbing procedure site, prior to antiseptic application.
Povidone Iodine Prep Swabstick - For antiseptic application at procedure site.
Stop Use
Stop use and ask a healthcare professional if;
- condition worsens or persists for more than 72 hours
- if pain, irritation, redness or swelling occurs
Keep Out Of Reach Of Children
- if swallowed, get medical help or contact a Poison Control Center immediately .
Dosage and Administration
Directions
For pre-operative patient skin preparation:
- Apply to procedure site and allow to dry.
- discard after single use.
- If not ready for immediate venipuncture, cover the area with a dry sterile gauze.
As a first aid antiseptic:
- clean affected area
- apply to wound once or twice daily
- may be covered with a sterile bandage
- if bandaged let dry first
Tear at notch, remove applicator, use only once.