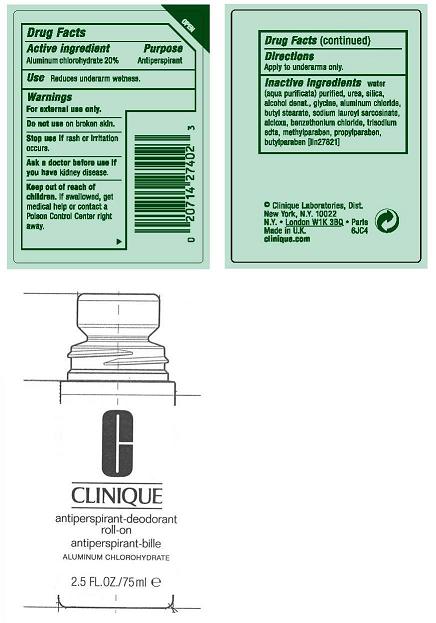
CLINIQUE ANTIPERSPIRANT DEODORANT ROLL-ON- aluminum chlorohydrate liquid
CLINIQUE LABORATORIES INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT:ALUMINUM CHLOROHYDRATE 20.00%
USES: DECREASES UNDERARM PERSPIRATION
WARNINGS:
- FOR EXTERNAL USE ONLY
- DO NOT USE ON BROKEN SKIN
- STOP USE IF RASH OR IRRITATION OCCURS
- ASK A DOCTOR BEFORE USE IF YOU HAVE KIDNEY DISEASE
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS: APPLY TO UNDERARMS ONLY.
inactive ingredients: water[] urea [] silica [] alcohol denat. [] glycine [] aluminum chloride [] butyl stearate [] sodium lauroyl sarcosinate [] alcloxa [] benzethonium chloride [] trisodium edta [] methylparaben [] propylparaben [] butylparaben iln27821
PRINCIPAL DISPLAY PANEL:
CLINIQUE
anti-perspirant deodorant roll-on
ALUMINUM CHLOROHYDRATE
2.5FL OZ./ 70ML
CLINIQUE LABORATORIES, DIST.
NEW YORK, NY 10022
6183
CLINIQUE.COM