Active Ingredients.....Purpose
Avobenzone 3% .................Sunscreen
Homosalate 12.5% .........… Sunscreen
Octisalate 5% ...............… Sunscreen
Octocrylene 8% ................. Sunscreen
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months of age: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Inactive Ingredients
Water, C12-15 Alkyl Benzoate, Butylene Glycol, Isododecane, Sorbitol, Silica, Styrene/Acrylates Copolymer, Cetearyl Alcohol, Niacinamide, Glycerin, Phenoxyethanol, Dimethicone, Glyceryl Stearate, PEG-100 Stearate, Ceteareth-20, Phenylethyl Resorcinol, Carbomer, Fatty Acid Ethoxylate, Sorbitan Stearate, Synthetic Fluorphlogopite, Ethylhexylglycerin, Titanium Dioxide, Disodium EDTA, Sodium Hydroxide, Tin Oxide
Principal Display Panel - 3 FL OZ Carton
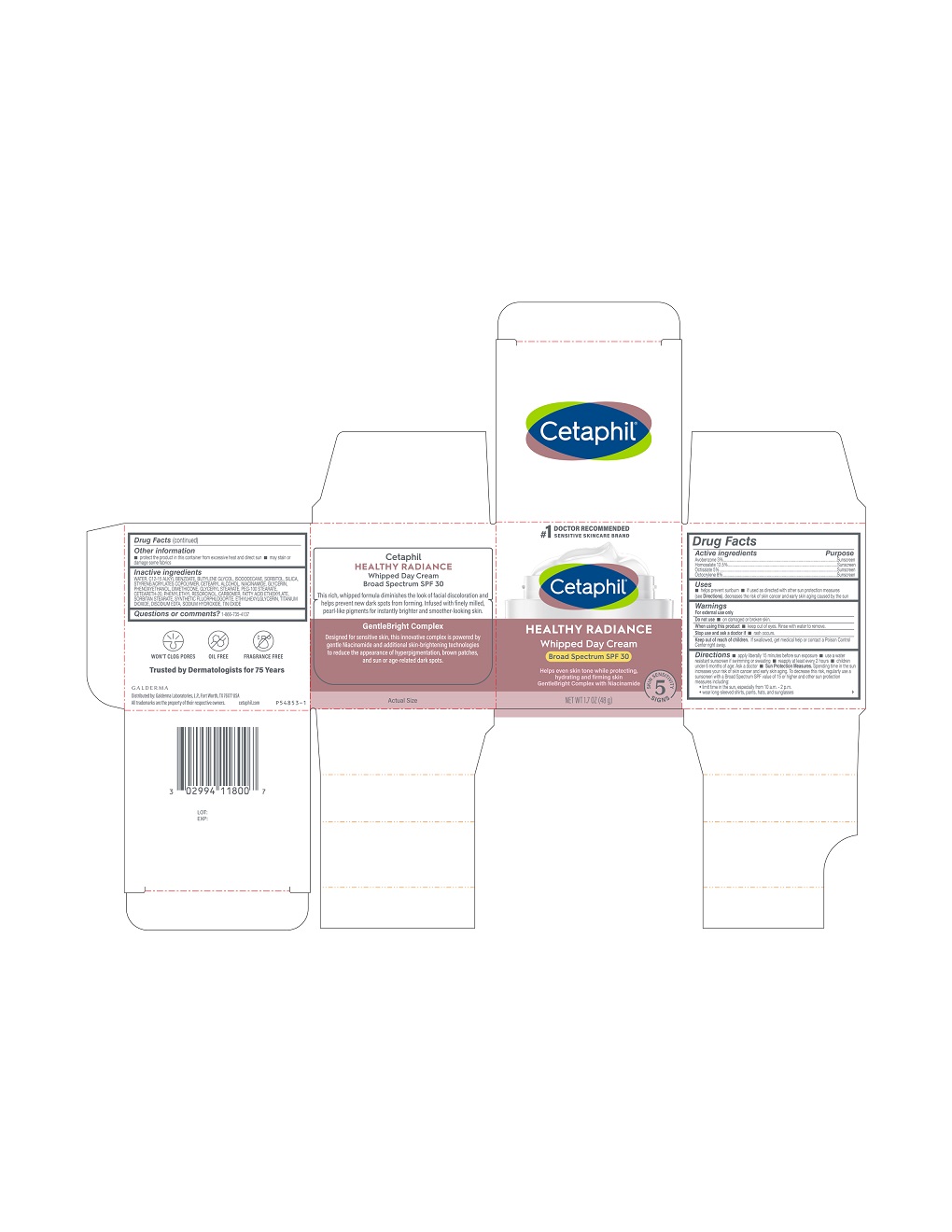
#1 Doctor Recommended
Sensitive Skincare Brand
Cetaphil®
HEALTHY RADIANCE
Whipped Day Cream
Broad Spectrum SPF 30
Helps even skin tone while protecting,
hydrating and firming skin
GentleBright Complex with Niacinamide
Five Skin Sensitivity Signs
NET WT 1.7 OZ (48 g)
Distributed by:
Galderma Laboratories, L.P., Fort Worth, TX 76177 USA
All trademarks are the property of their respective owners.
Cetaphil.com
P54853-1