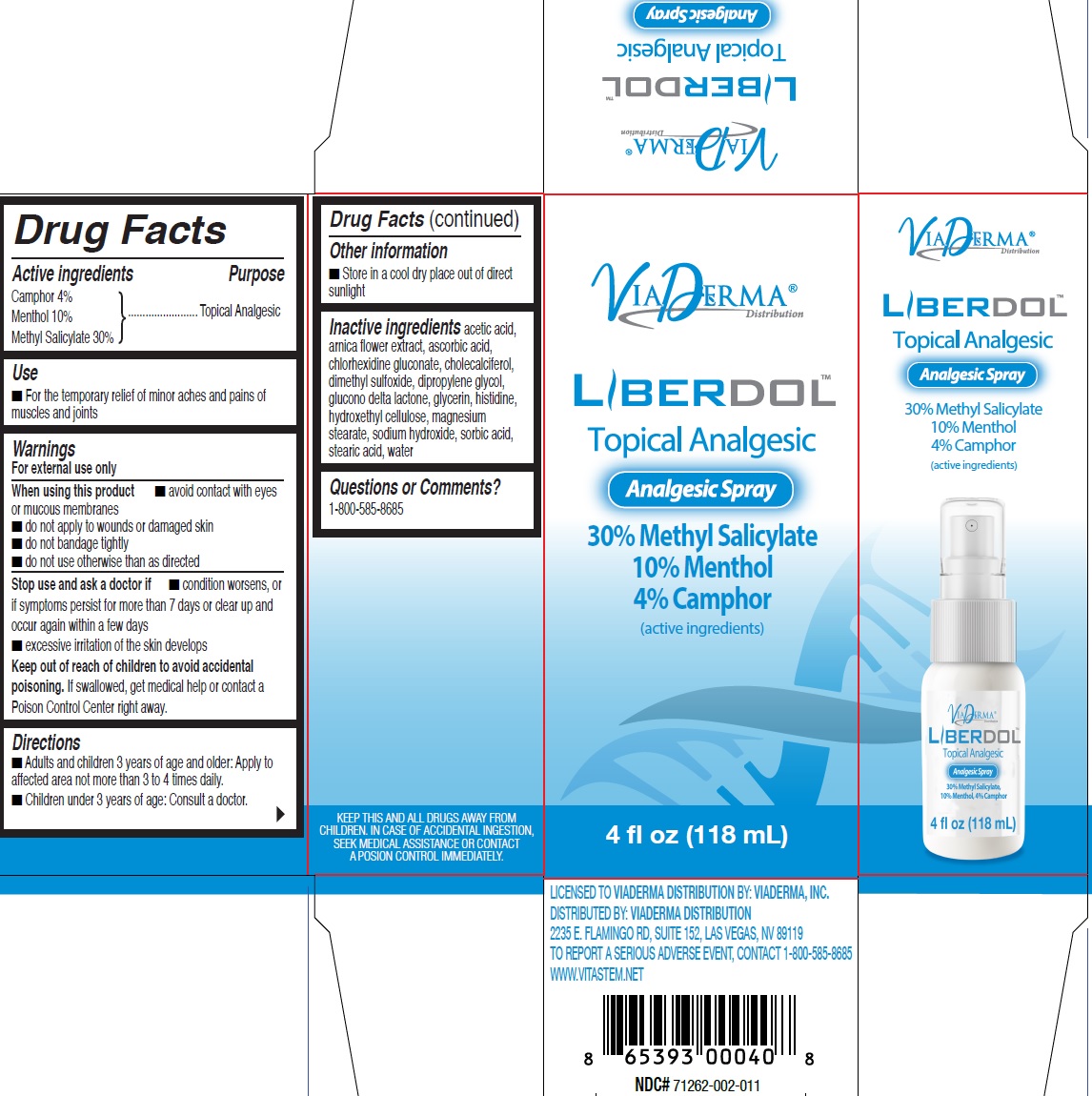
LIBERDOL ANALGESIC- menthol, unspecified form, methyl salicylate liquid
ViaDerma Distribution, Inc.
----------
Active ingredients
Camphor 4% Menthol 10%
Methyl Salicylate 30%
Purpose
Topical Analgesic
Use
- For the temporary relief of minor aches and pains of muscles and joints
Warnings
For external use only
When using this product
- avoid contact with eyes or mucous membranes
- do not apply to wounds or damaged skin
- do not bandage tightly
- do not use otherwise than as directed
Stop use and ask a doctor if
- condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days
- excessive irritation of the skin develops
Keep out of reach of children to avoid accidental poisoning.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 3 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- Children under 3 years of age: Consult a doctor.
Other information
- Store in a cool dry place out of direct sunlight
Inactive ingredients
acetic acid, arnica flower extract, ascorbic acid, chlorhexidine gluconate, cholecalciferol, dimethyl sulfoxide, dipropylene glycol, glucono delta lactone, glycerin, histidine, hydroxethyl cellulose, magnesium stearate, sodium hydroxide, sorbic acid, stearic acid, water
Questions or Comments?
1-800-585-8685
Package Labeling:

ViaDerma Distribution, Inc.

