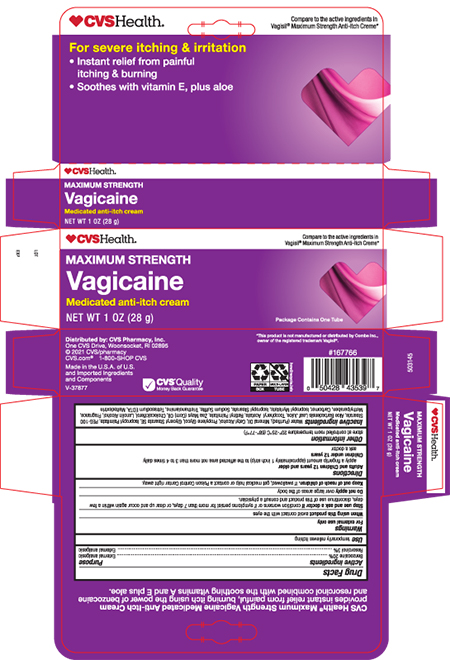
Warnings
For external use only
Allergy Allert
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other –caine anesthetics.
Avoid contact with eyes
in case of contact rinse thoroughly and immediately with water.
Stop use and ask doctor if
condition worsens, or if symptoms persist for more than 7 days, or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years and older
Apply a finger tip amount (approxiamtely a 1 inch strip) to the affected areas not more than 3 to 4 times daily
Children under 12 years
ask a doctor
Inactive ingredients
Water, Mineral Oil, Cetyl Alcohol, Propylene Glycol, Glyceryl Stearate, PEG-100 Stearate, Isopropyl Palmitate, Aloe Barbadensis Leaf Extract, Tocopheryl Acetate, Retinyl Palmitate, Zea Mays (Corn) Oil, Cholecalciferol, Lanolin Alcohol, Fragrance, Methylparaben, Carbomer, Isopropyl Myristate, Isopropyl Stearate, Sodium Sulfite, Triethanolamine, Trisodium EDTA, Maltodextrin