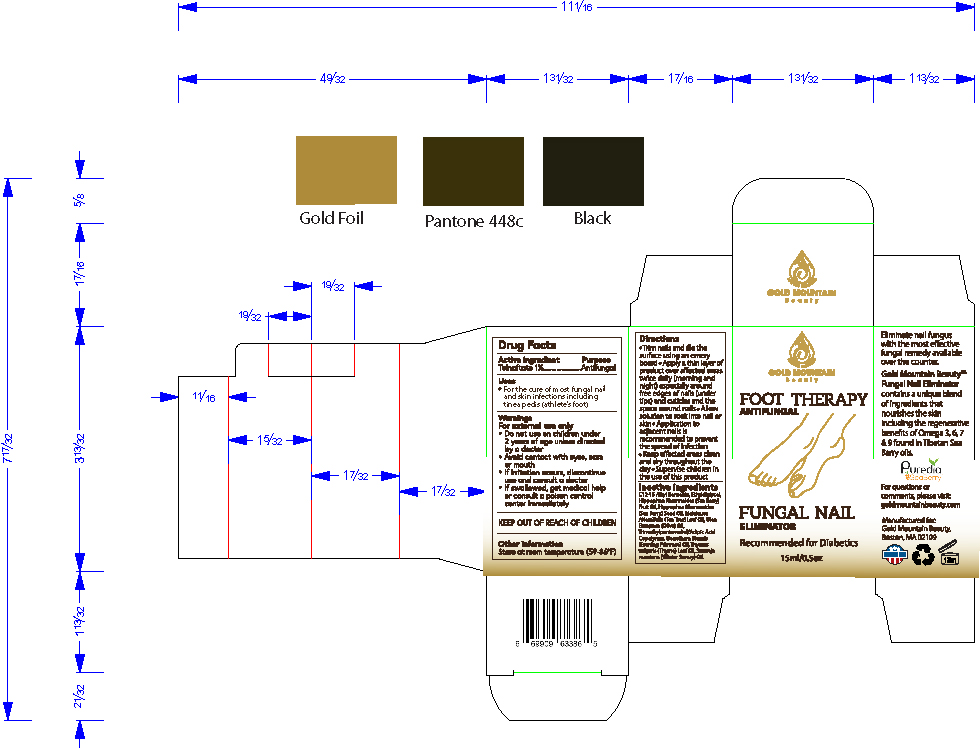
For external use only
- Do not use on children under 2 years of age unless directed by a doctor
- Avoid contact with eyes, ears or mouth
- If irritation occurs, discontinue use and consult a doctor
- If swallowed, get medical help or consult a poison control center immediately
Inactive ingredients
C12-15 Alkyl Benzoate, Ethyldiglycol, Hippophae Rhamnoides (Sea Berry) Fruit Oil, Hippophae Rhamnoides (Sea Berry) Seed Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Olea Europaea (Olive) Oil, Trimethylpentanediol/Adipic Acid Copolymer
Trim nails and file the surface using an emery board
Apply a thin layer of product over affected areas twice daily (morning and night) especially around free edges of nails (under tips) and cuticles and the space around nails
Allow solution to soak into nail or skin
Application to adjacent nails is recommended to prevent the spread of infection
Keep affected areas clean and dry throughout the day
Supervise children in the use of this product
Warnings
For external use only
- Do not use on children under 2 years of age unless directed by a doctor
- Avoid contact with eyes, ears or mouth
- If irritation occurs, discontinue use and consult a doctor
- If swallowed, get medical help or consult a poison control center immediately.
Directions
- Trim nails and file the surface using an emery board
- Apply a thin layer of product over affected areas twice daily (morning and night) especially around free edges of nails (under tips) and cuticles and the space around nails
- Allow solution to soak into nail or skin
- Application to adjacent nails is recommended to prevent the spread of infection
- Keep affected areas clean and dry throughout the day
- Supervise children in the use of this product
 GOLD MOUNTAIN
GOLD MOUNTAIN