PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Pemetrexed for Injection, USP
100 mg/vial
For intravenous use only.
Single-Dose Vial
Rx only

Pemetrexed for Injection, USP
100 mg/vial
For intravenous use only.
One Single-Dose Vial Carton
Rx only

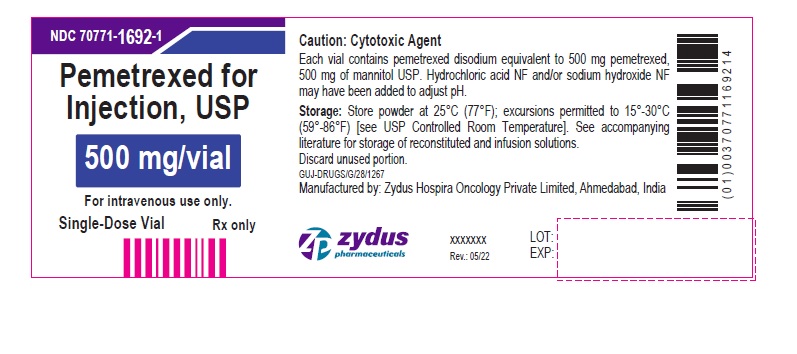
Pemetrexed for Injection, USP
500 mg/vial
For intravenous use only.
Single-Dose Vial
Rx only
Pemetrexed for Injection, USP
500 mg/vial
For intravenous use only.
One Single-Dose Vial Carton
Rx only

Pemetrexed for Injection, USP
1000 mg/vial
For intravenous use only.
Single-Dose Vial
Rx only

Pemetrexed for Injection, USP
1000 mg/vial
For intravenous use only.
One Single-Dose Vial Carton
Rx only
