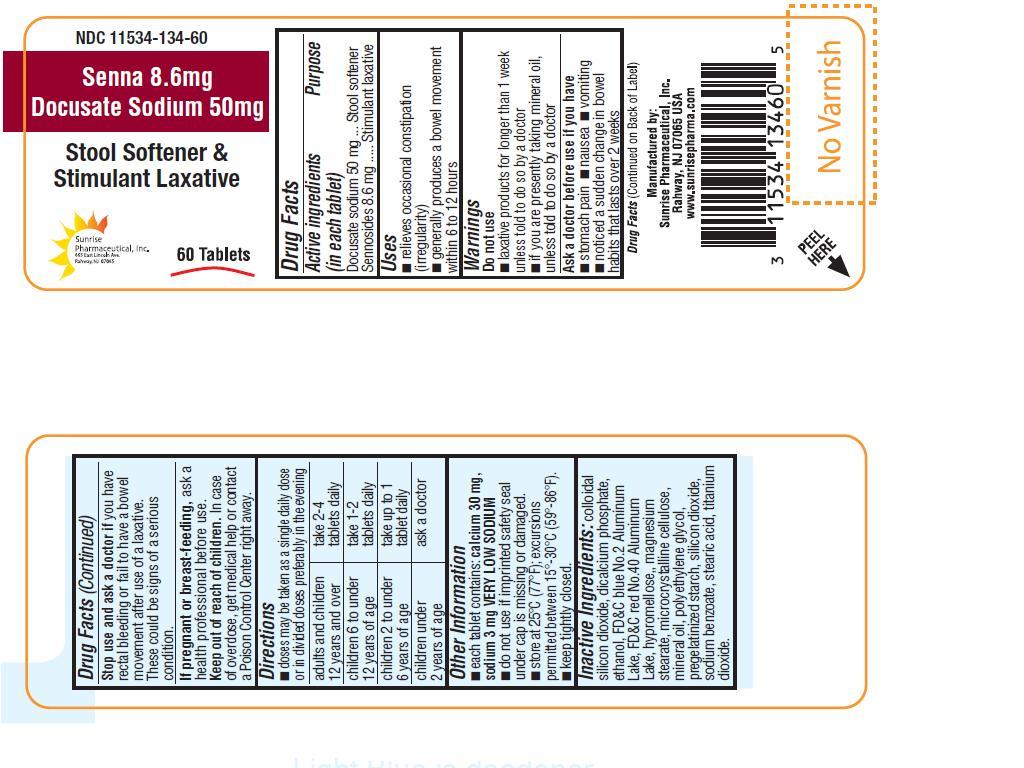
INDICATIONS AND USAGE
For temporary relief of occasional constipation and irregularity.
This product generally produces bowel movement in 6 to 12 hours.
WARNINGS
Do not use:
laxative products for longer than 1 week unless told to do so by a doctor.
if you are presently taking mineral oil, unless told to do so by a doctor
OTC - ASK DOCTOR
Before use if you have:
Stomach pain, nausea or vomiting
Noticed a sudden change in bowel habits that lasts over 2 weeks.
OTC - STOP USE
If you have rectal bleeding or fail to have bowel movement after use of a laxative. This could be a serious condition.
OTC - KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE AND ADMINISTRATION
Drink with a full glass of water with each dose
| Adults and children 12 years and over | take 2 - 4 tablets daily |
| Children 6 to under 12 years | take 1 - 2 tablets daily |
| Children 2 to under 6 years | take up to 1 tablet daily |
| Children under 2 years of age | Ask a doctor |
OTHER INFORMATION
Each tablet contains: calcium 30 mg, sodium 3 mg VERY LOW SODIUM.
Do not use if the imprinted safety seal under cap is missing or damaged.
Store at at 25°C (77°F); excursions permitted between 15(-30(C (59(-86(F)
Keep tightly closed.