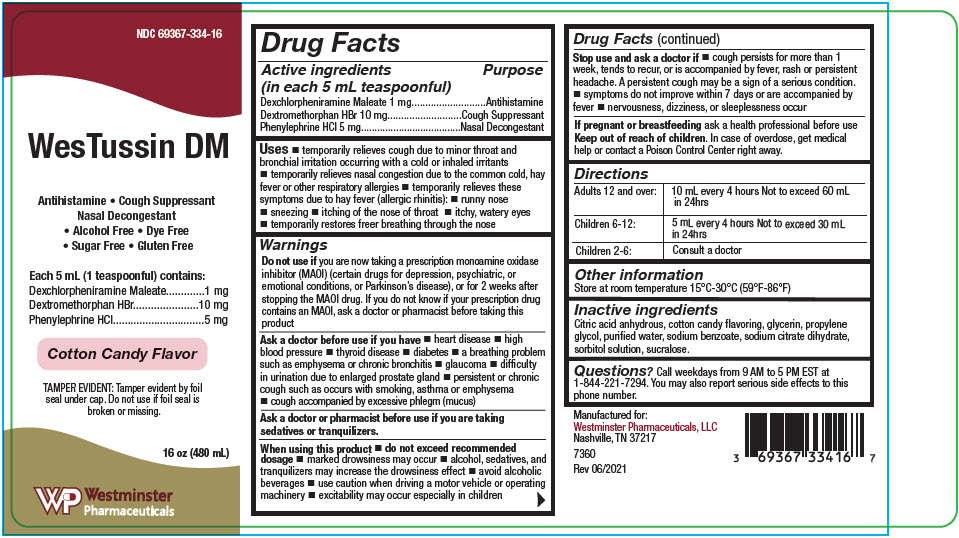
Uses
- temporarily relieves cough due to minor throat and bronchial irritation occurring with a cold or inhaled irritants
- temporarily relieves nasal congestion due to the common cold, hay fever or other respiratory allergies
- temporarily relieves these symptoms due to hay fever (allergic rhinitis):
- runny nose
- sneezing
- itching of the nose of throat
- itchy, watery eyes
- temporarily restores freer breathing through the nose
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma or emphysema
- cough accompanied by excessive phlegm (mucus)
When using this product
- do not exceed recommended dosage
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase the drowsiness effect
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
- excitability may occur especially in children
Stop use and ask a doctor if
- cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash or persistent headache. A persistent cough may be a sign of a serious condition.
- symptoms do not improve within 7 days or are accompanied by fever
- nervousness, dizziness, or sleeplessness occur
Directions
| Adults 12 and over: | 10 mL every 4 hours Not to exceed 60 mL in 24hrs |
| Children 6-12: | 5 mL every 4 hours Not to exceed 30 mL in 24hrs |
| Children 2-6: | Consult a doctor |
Inactive ingredients
Citric acid anhydrous, cotton candy flavoring, glycerin, propylene glycol, purified water, sodium benzoate, sodium citrate dihydrate, sorbitol solution, sucralose.
Questions?
Call weekdays from 9 AM to 5 PM EST at 1-844-221-7294. You may also report serious side effects to this phone number.
PRINCIPAL DISPLAY PANEL - 480 mL Bottle Label
NDC 69367-334-16
WesTussin DM
Antihistamine • Cough Suppressant
Nasal Decongestant
• Alcohol Free • Dye Free
• Sugar Free • Gluten Free
Each 5 mL (1 teaspoonful) contains:
Dexchlorpheniramine Maleate
1 mg
Dextromethorphan HBr
10 mg
Phenylephrine HCl
5 mg
Cotton Candy Flavor
TAMPER EVIDENT: Tamper evident by foil
seal under cap. Do not use if foil seal is
broken or missing.
16 oz (480 mL)
Westminster
Pharmaceuticals