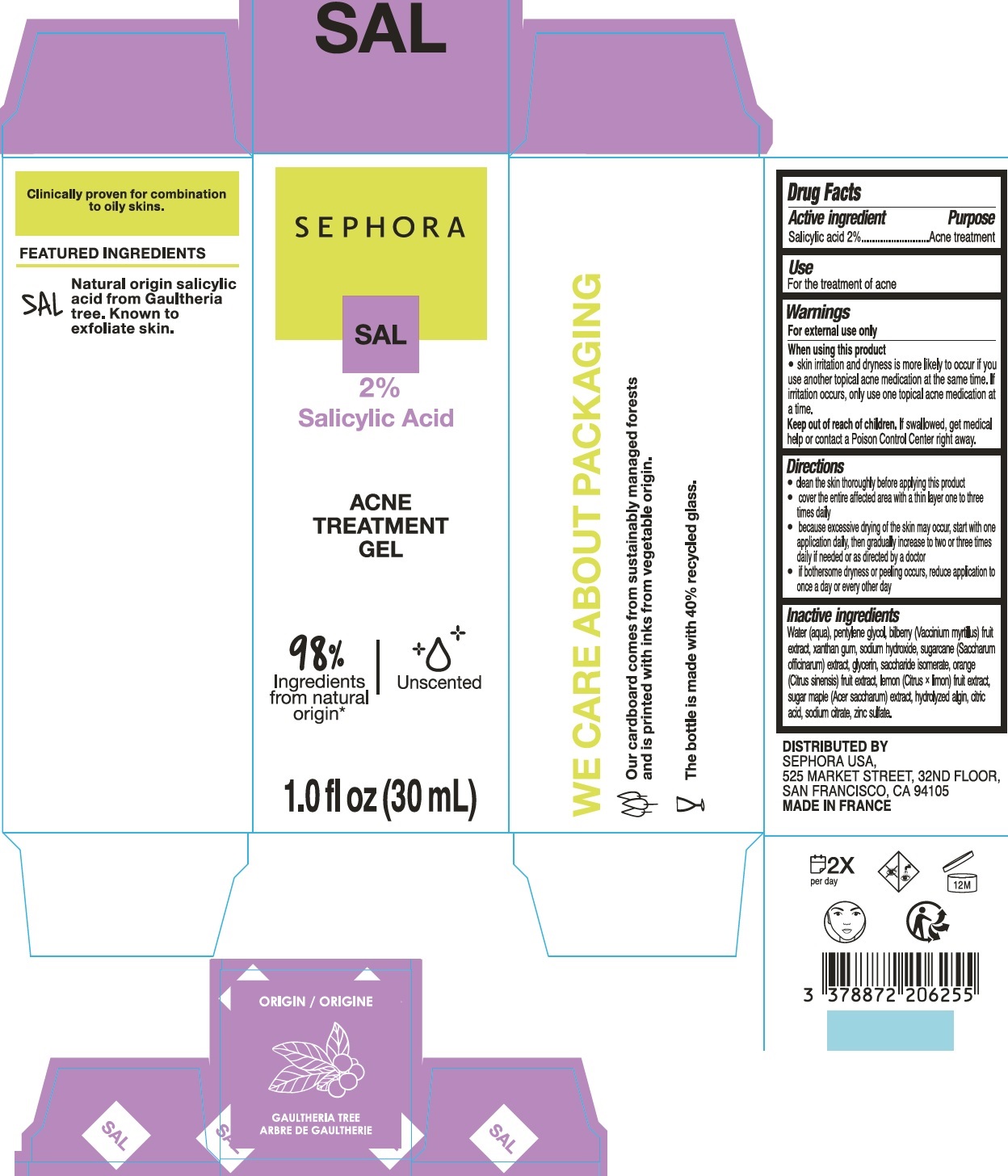
Warnings
For external use only
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
Water (aqua), pentylene glycol, biberry (Vaccinium myrtilus) fruit extract, xanthan gum, sodium hydroxide, sugarcane (Saccharum officinarum) extract, glycerin, saccharide isomerate, orange (Citrus sinensis) fruit extract, lemon (Citrus x limon) fruit extract, sugar maple (Acer saccharum) extract, hydrolyzed algin, citric acid, sodium citrate, zinc sulfate.