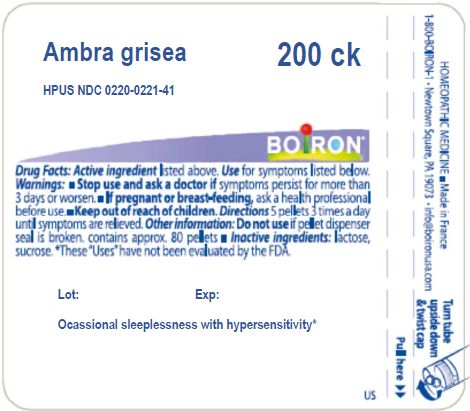
AMBRA GRISEA- ambergris pellet
Boiron
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Ambra grisea
| AMBRA GRISEA
ambergris pellet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Boiron (282560473) |
| Registrant - Boiron, Inc. (014892269) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boiron | 282560473 | manufacture(0220-0221) | |
Revised: 11/2020
Document Id: b325563c-b72b-21ff-e053-2995a90a0216
Set id: 687e64ac-7270-c2b2-e053-2a91aa0a1f4e
Version: 3
Effective Time: 20201102
Boiron