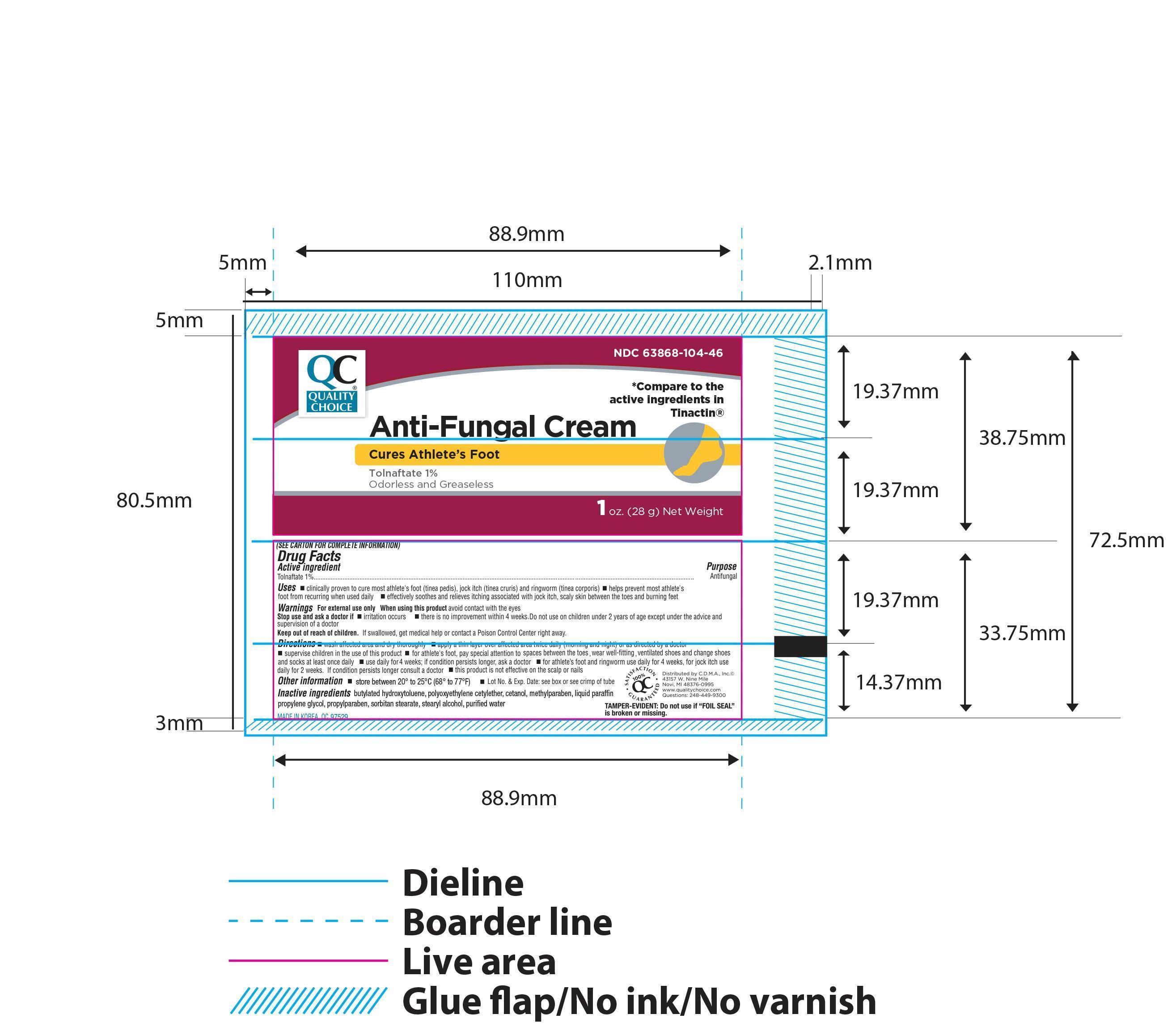
Active ingredient Purpose
Tolnaftate 1%....................................................................Antifungal
Uses
- clinically proven to cure most athlete's foot (tinea pedis), jock itch (tinea cruris) and ringworm (tinea corporis)
- helps prevent most athlete's foot recurring when used daily
- effectively soothes and relieves itching associated with jock itch, scaly skin between the toes and burning feet
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product for athlete's foot, pay special attention to space between the toes, wear well-fitting, ventilated shoes and change shoes and socks at least one daily
- use daily for 4 weeks; if condition persists longer, ask a doctor
- for athlete's foot and ringworm use daily for 4 weeks, for jock itch use daily for 2 weeks. If condition persists longer consult a doctor
- this product is not effective on the scalp or nails
Other information
- store between 2° and 30° C (36° and 86° F)
- Lot No. and Exp. Date:see box or see crimp of tube