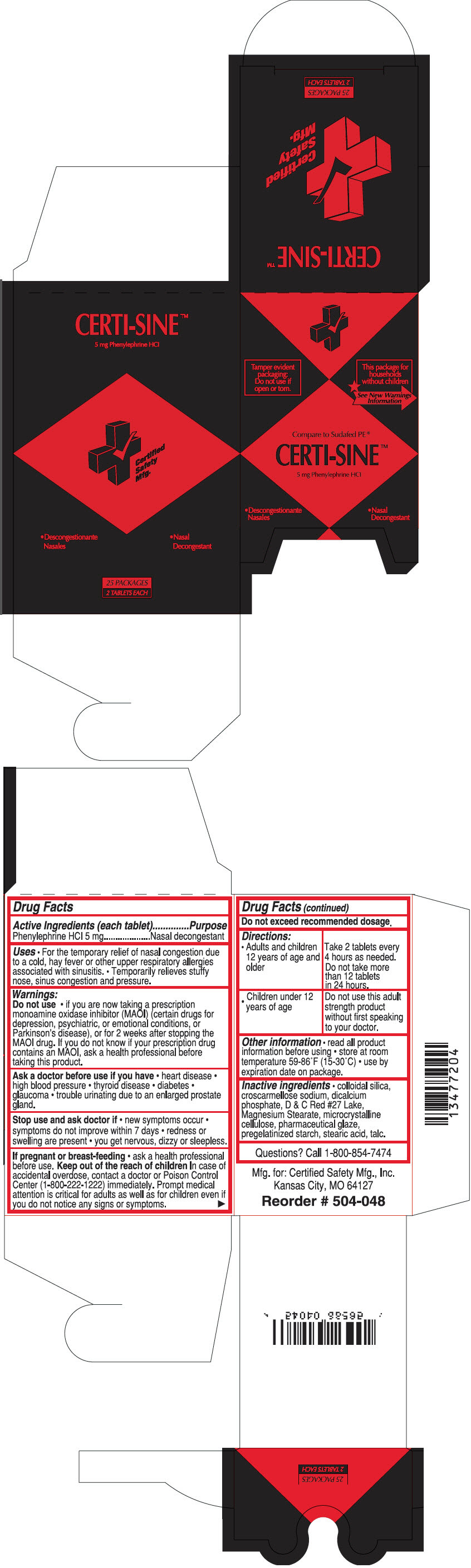
CERTI-SINE- phenylephrine hydrochloride tablet
Certified Safety Manufacturing
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients (each tablet)
Phenylephrine HCl 5 mg
Purpose
Nasal decongestant
Uses
- For the temporary relief of nasal congestion due to a cold, hay fever or other upper respiratory allergies associated with sinusitis.
- Temporarily relieves stuffy nose, sinus congestion and pressure.
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a health professional before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland.
Stop use and ask a doctor if
- new symptoms occur
- symptoms do not improve within 7 days
- redness or swelling are present
- you get nervous, dizzy or sleepless.
If pregnant or breast-feeding
- ask a health professional before use.
Keep out of the reach of children. In case of accidental overdose, contact a doctor or Poison Control Center (1-800-222-1222) immediately. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Do not exceed recommended dosage.
Directions
-
Adults and children 12 years of age and older
| Take 2 tablets every 4 hours as needed.
Do not take more than 12 tablets in 24 hours. |
-
Children under 12 years of age
| Do not use this adult strength product without first speaking to your doctor. |
Other information
- read all product information before using
- store at room temperature 59-86°F (15-30°C)
- use by expiration date on package.
Inactive ingredients
- Colloidal silica, croscarmellose sodium, dicalcium phosphate, D & C Red #27 Lake, Magnesium Stearate, microcrystalline cellulose, pharmaceutical glaze, pregelatinized starch, stearic acid, talc.
Questions?
Call 1-800-854-7474
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Pouch Box
Tamper evident
packaging:
Do not use if
open or torn.
This package for
households
without children
See New Warnings
Information
Compare to Sudafed PE ®
CERTI-SINE™
5 mg Phenylephrine HCl