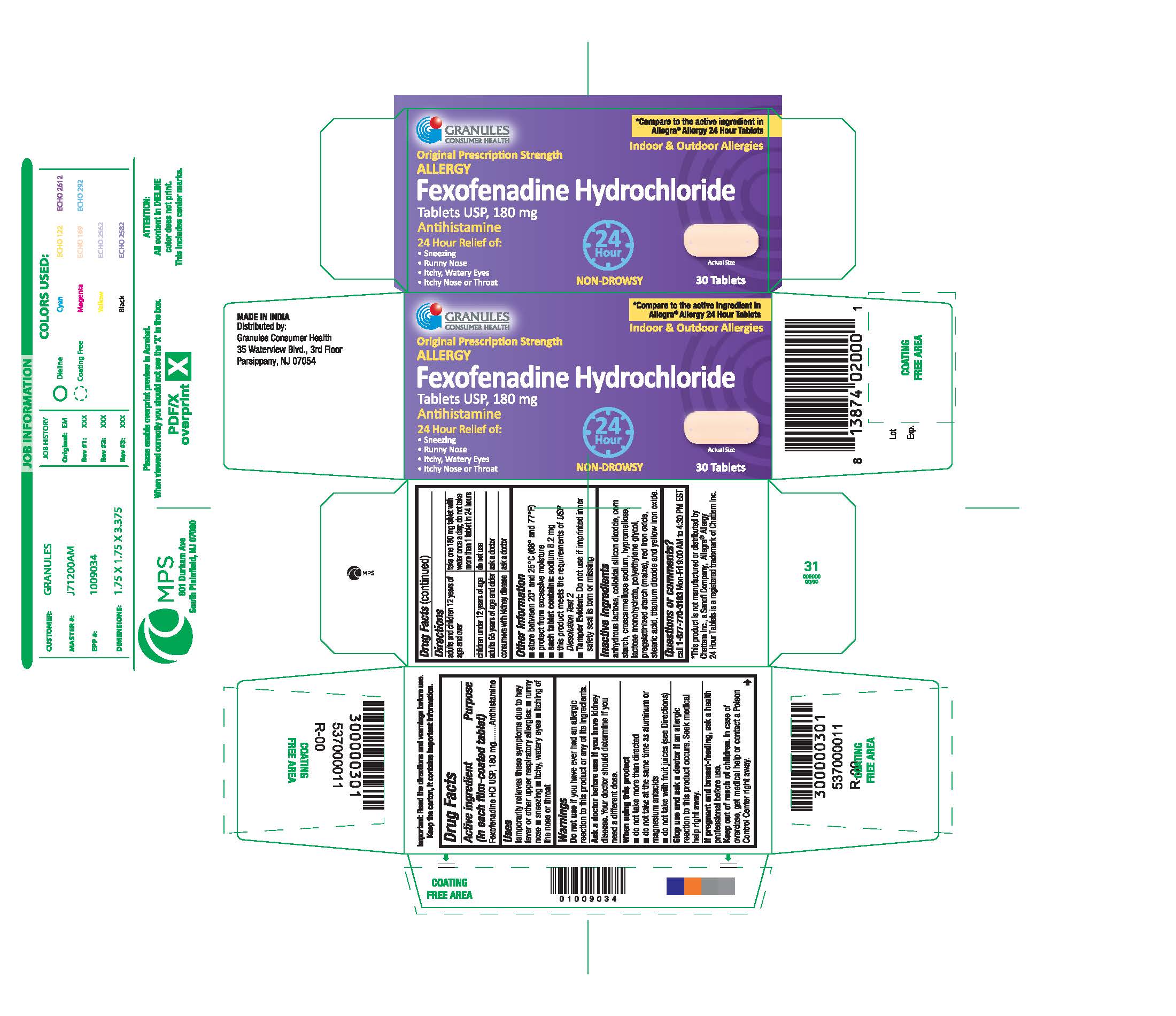
FEXOFENADINE HYDROCHLORIDE- fexofenadine hydrochloride tablet, film coated
Granules USA, Inc
----------
Original Prescription Strength
ALLERGY
Fexofenadine Hydrochloride
Tablets USP, 180 mg
Antihistamine
24 Hour Relief of
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
■ runny nose
■ sneezing
■ itchy, watery eyes
■ itching of the nose or throat
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
■ do not take more than directed
■ do not take at the same time as aluminum or magnesium antacids
■ do not take with fruit juices (see Directions)
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
adults and children 12 years of age and over: take one 180mg tablet with water once a day
do not take more than 1 tablet in 24 hours
children under 12 years of age: do not use
adults 65 years of age and older: ask a doctor
consumers with kidney disease: ask a doctor
Other information
■ store between 20°and 25°C (68°and 77°F)
■ protect from excessive moisture
■ each tablet contains: sodium 8.2 mg
■ this product meets the requirements of USP Dissolution Test 2
■ Tamper Evident: Do not use if imprinted inner safety seal is torn or missing
| FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Granules USA, Inc (137098864) |