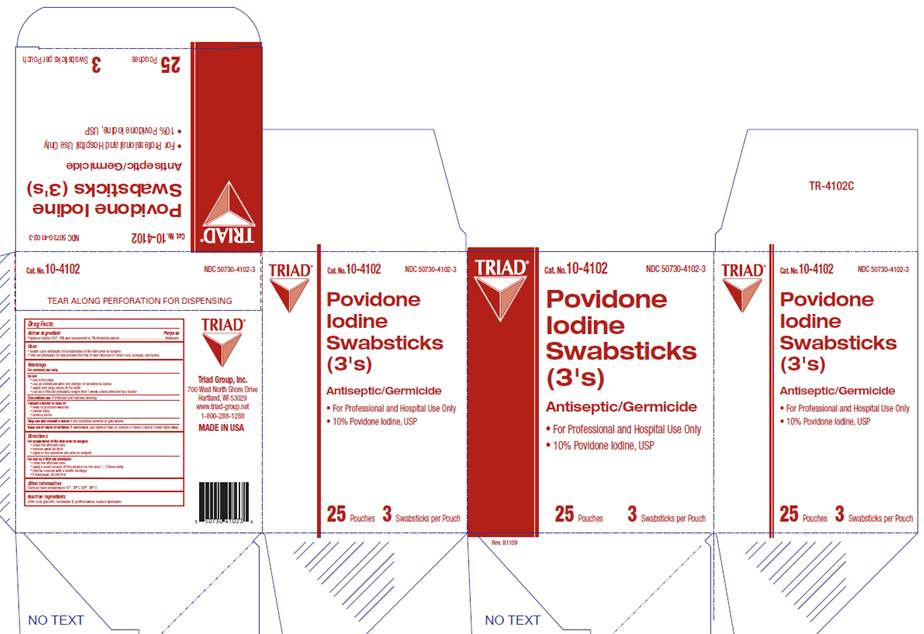
POVIDONE-IODINE PREP SWABSTICKS - povidone-iodine solution
Triad Group
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
Povidone Iodine USP 10% w/v (equivalent to 1% titratable iodine)
USES
- Health Care Antiseptic for preparation of the skin prior to surgery
- First Aid Antiseptic to help prevent the risk of infection in minor cuts, scrapes and burns
WARNINGS
For external use only.
Do not
- use in the eyes
- apply over large areas of the body
- use on individuals who are allergic or sensitive to iodine
- use as a first aid antiseptic for longer than 1 week unless directed by a doctor
Ask a doctor in case of
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor
- if condition persists for more than 72 hours
- if irritation and redness develop
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
For preparation of the skin prior to surgery
- clean the affected area
- remove swab by stick
- apply to the operative site prior to surgery
As a first aid antiseptic
- clean the affected area
- apply a small amount of this product to the area 1-3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
OTHER INFORMATION
Store at room temperature 15° - 30° C (59° - 86° F)
INACTIVE INGREDIENTS
citric acid, glycerin, nonoxynol-8, purified water, sodium hydroxide
POUCH INFORMATION
TRIAD
Cat. No. 10-4102
NDC 50730-4102-3
10% Povidone Iodine, USP
POVIDONE IODINE
SWABSTICKS (3's)
Antiseptic / Germicide
10% Povidone Iodine, USP
For Hospital and Professional Use Only
Contents:
3 Swabsticks
Triad Group, Inc.
700 West North Shore Drive
Hartland, WI 53029
MADE IN USA
www.triad-group.net
CARTON INFORMATION
TRIAD
Cat. No. 10-4102
NDC 50730-4102-3
POVIDONE IODINE
SWABSTICKS (3's)
Antiseptic / Germicide
For Hospital and Professional Use
10% Povidone Iodine, USP
25 Pouches
3 Swabsticks per Pouch