FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Flublok Quadrivalent is a vaccine indicated for active immunization against disease caused by influenza A subtype viruses and type B viruses contained in the vaccine. Flublok Quadrivalent is approved for use in persons 18 years of age and older [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.2 Administration
Invert the prefilled syringe containing Flublok Quadrivalent gently prior to affixing the appropriate size needle for intramuscular administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
The preferred site for injection is the deltoid muscle. Flublok Quadrivalent should not be mixed in the same syringe with any other vaccine.
3 DOSAGE FORMS AND STRENGTHS
Flublok Quadrivalent is a sterile solution supplied in prefilled, single-dose syringes, 0.5 mL.
4 CONTRAINDICATIONS
Flublok Quadrivalent is contraindicated in individuals with known severe allergic reactions (e.g., anaphylaxis) to any component of the vaccine [see Postmarketing Experience (6.2) and Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Managing Allergic Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
5.2 Guillain Barré Syndrome
The 1976 swine influenza vaccine was associated with an increased frequency of Guillain-Barré Syndrome (GBS). Evidence for a causal relation of GBS with other influenza vaccines is inconclusive; if an excess risk exists, it is probably slightly more than one additional case per 1 million persons vaccinated. If GBS has occurred within 6 weeks of receipt of a prior influenza vaccine, the decision to give Flublok should be based on careful consideration of the potential benefits and risks.
6 ADVERSE REACTIONS
In adults 18 through 49 years of age, the most common (≥10%) injection-site reactions were tenderness (48%) and pain (37%); the most common (≥10%) solicited systemic adverse reactions were headache (20%), fatigue (17%), myalgia (13%), and arthralgia (10%) [see Clinical Trials Experience (6.1)].
In adults 50 years of age and older, the most common (≥10%) injection site reactions were tenderness (34%) and pain (19%); the most common (≥10%) solicited systemic adverse reactions were headache (13%) and fatigue (12%) [see Clinical Trials Experience (6.1)].
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a vaccine cannot be directly compared to rates in the clinical studies of another vaccine and may not reflect the rates observed in clinical practice.
Flublok Quadrivalent
Flublok Quadrivalent has been administered to and safety data collected from 998 adults 18-49 years of age (Study 1) and 4328 adults 50 years of age and older (Study 2).
In Studies 1 and 2, local (injection site) and systemic adverse reactions were solicited with the use of a memory aid for 7 days following vaccination, unsolicited adverse events were collected for ~28 days post-vaccination, and serious adverse events (SAEs) were collected for 6 months post-vaccination via clinic visit or remote contact.
Study 1 included 1330 subjects 18 through 49 years of age for safety analysis, randomized to receive Flublok Quadrivalent (n=998) or a comparator inactivated influenza vaccine (Fluarix® Quadrivalent, manufactured by GlaxoSmithKline) (n=332) [see Clinical Studies (14)]. The mean age of participants was 33.5 years. Overall, 65% of subjects were female, 59% white/Caucasian, 37% black/African American, 1.0% Native Hawaiian/Pacific Islander, 0.8% American Indian/Alaskan Native, 0.5% Asian, 1.4% other racial groups, and 16% of Hispanic/Latino ethnicity. Table 1 summarizes the incidence of solicited local and systemic adverse reactions reported within seven days of vaccination with Flublok Quadrivalent or the comparator vaccine.
| Reactogenicity Term | Flublok Quadrivalent N=996 % | Comparator N=332 % |
||||
|---|---|---|---|---|---|---|
| Any Grade‡ | Grade 3 | Grade 4 | Any Grade‡ | Grade 3 | Grade 4 | |
| NOTE: Data based on the most severe response reported by subjects. Results ≥1% reported to nearest whole percent; results >0 but <1% reported as <1%. | ||||||
|
||||||
| Subjects with ≥1 injection site reaction§,¶ | 51 | 1 | 0 | 52 | 2 | 0 |
| Local Tenderness | 48 | 1 | 0 | 47 | 1 | 0 |
| Local Pain | 37 | 1 | 0 | 36 | 1 | 0 |
| Firmness / Swelling | 5 | 0 | 0 | 3 | 0 | 0 |
| Redness | 4 | 0 | 0 | 1 | 0 | 0 |
| Subjects with ≥1 systemic reaction§,# | 34 | 2 | <1 | 36 | 3 | <1 |
| Headache | 20 | 1 | 0 | 21 | 2 | <1 |
| Fatigue | 17 | 1 | 0 | 17 | 1 | 0 |
| Muscle Pain | 13 | 1 | 0 | 12 | 1 | 0 |
| Joint Pain | 10 | 1 | 0 | 10 | 1 | 0 |
| Nausea | 9 | 1 | <1 | 9 | 1 | 0 |
| Shivering / Chills | 7 | 1 | 0 | 6 | 1 | 0 |
| Fever‡,Þ | 2 | <1 | 0 | 1 | <1 | 0 |
Study 2 included 8672 subjects 50 years of age and older for safety analysis, randomized to receive Flublok Quadrivalent (n=4328) or Comparator (Fluarix Quadrivalent, manufactured by GlaxoSmithKline) as an active control (n=4344) [see Clinical Studies (14)]. The mean age of participants was 62.7 years. Overall, 58% of subjects were female, 80% white/Caucasian, 18% black/African American, 0.9% American Indian/Alaskan Native, 0.4% Asian, 0.2% Native Hawaiian/Pacific Islander, 0.7% other racial groups, and 5% of Hispanic/Latino ethnicity. Table 2 summarizes the incidence of solicited local and systemic adverse reactions reported within seven days of vaccination with Flublok Quadrivalent or Comparator.
| Reactogenicity Term | Flublok Quadrivalent N=4312 % | Comparator N=4327 % |
||||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3 | Grade 4 | Any Grade | Grade 3 | Grade 4 | |
| NOTE: Data based on the most severe response reported by subjects. Results ≥1% reported to nearest whole percent; results >0 but <1% reported as <1%. | ||||||
|
||||||
| Subjects with ≥1 injection site reaction‡,§ | 38 | <1 | <1 | 40 | <1 | <1 |
| Local Tenderness | 34 | <1 | <1 | 37 | <1 | <1 |
| Local Pain | 19 | <1 | 0 | 22 | <1 | <1 |
| Firmness / Swelling | 3 | <1 | 0 | 3 | <1 | 0 |
| Redness | 3 | <1 | 0 | 2 | <1 | 0 |
| Subjects with ≥1 systemic reactogenicity event‡,¶ | 25 | 1 | <1 | 26 | 1 | <1 |
| Headache | 13 | <1 | <1 | 14 | 1 | <1 |
| Fatigue | 12 | <1 | 0 | 12 | <1 | <1 |
| Muscle Pain | 9 | <1 | <1 | 9 | <1 | <1 |
| Joint Pain | 8 | <1 | 0 | 8 | <1 | <1 |
| Nausea | 5 | <1 | 0 | 5 | <1 | <1 |
| Shivering / Chills | 5 | <1 | 0 | 4 | <1 | <1 |
| Fever#,Þ | <1 | <1 | 0 | 1 | <1 | 0 |
Among adults 18-49 years of age (Study 1), through 6 months post-vaccination, no deaths were reported. SAEs were reported by 12 subjects, 10 (1%) Flublok Quadrivalent recipients and 2 (0.6%) Comparator recipients. No SAEs were considered related to study vaccine.
Among adults 50 years of age and older (Study 2), 20 deaths occurred in the 6 months post-vaccination, including 8 Flublok Quadrivalent and 12 Comparator recipients. No deaths were considered related to study vaccine. SAEs were reported by 145 (3.4%) Flublok Quadrivalent recipients and 132 (3%) Comparator recipients. No SAEs were considered related to study vaccine.
In the 28 days following vaccination, one or more unsolicited treatment emergent adverse events occurred in 10.3% of Flublok Quadrivalent and 10.5% of Comparator recipients in Study 1 (adults 18-49 years of age) and in 13.9% of Flublok Quadrivalent and 14.1% of Comparator recipients in Study 2 (adults ≥50 years of age). In both studies, rates of individual events were similar between treatment groups, and most events were mild to moderate in severity.
Flublok (Trivalent Formulation)
The safety experience with Flublok is relevant to Flublok Quadrivalent because both vaccines are manufactured using the same process and have overlapping compositions [see Description (11)].
Flublok (trivalent formulation) has been administered to and safety data collected from a total of 4547 subjects in five clinical trials (Studies 3-7): 2497 adults 18 through 49 years, 972 adults 50 through 64 years, and 1078 adults 65 years and older. In Studies 3 - 5 and 7, SAEs were collected for 6 months post-vaccination. Study 6 collected SAEs through 30 days following receipt of vaccine. Study 6 also actively solicited pre-specified common hypersensitivity-type reactions through 30 days following receipt of vaccine as a primary endpoint.
Study 3 included 4648 subjects 18 through 49 years of age for safety analysis, randomized to receive Flublok (n=2344) or placebo (n=2304) [see Clinical Studies (14)].
Study 4 included 602 subjects 50 through 64 years of age for safety analysis, randomized to receive Flublok (n=300) or another U.S.–licensed trivalent influenza vaccine (Fluzone®, manufactured by Sanofi Pasteur, Inc.) as an active control (n=302).
Study 5 included 869 subjects aged 65 years and older for safety analysis, randomized to receive Flublok (n=436) or another U.S.–licensed trivalent influenza vaccine (Fluzone) as an active control (n=433).
Study 6 included 2627 subjects aged 50 years and older for safety analysis, randomized to receive Flublok (n=1314) or another U.S.–licensed trivalent influenza vaccine (Afluria, manufactured by Seqirus Pty Ltd.) as an active control (n=1313). Among subjects 50 through 64 years of age, 672 received Flublok and 665 received Afluria. Among subjects aged 65 years and older, 642 received Flublok and 648 received Afluria.
Study 7 was a Phase 2 dose-finding trial conducted in adults 18 through 49 years of age, 153 of whom received Flublok 135 mcg, the licensed trivalent formulation.
Serious Adverse Events
Among 2497 adults 18-49 years of age (Studies 3 and 7 pooled), through 6 months post-vaccination, two deaths were reported, one in a Flublok recipient and one in a placebo recipient. Both deaths occurred more than 28 days following vaccination and neither was considered vaccine-related. SAEs were reported by 32 Flublok recipients and 35 placebo recipients. One SAE (pleuropericarditis) in a Flublok recipient was assessed as possibly related to the vaccine.
Among 972 adults 50-64 years of age (Studies 4 and 6 pooled), through up to 6 months post-vaccination, no deaths occurred, and SAEs were reported by 10 subjects, 6 Flublok recipients and 4 Comparator recipients. One of the SAEs, vasovagal syncope following injection of Flublok, was considered related to administration of study vaccine.
Among 1078 adults 65 years of age and older (Studies 5 and 6 pooled), through up to 6 months post-vaccination, 4 deaths occurred, 2 in Flublok recipients and 2 in Comparator recipients. None were considered related to the study vaccines. SAEs were reported by 80 subjects (37 Flublok recipients, 43 Comparator recipients). None were considered related to the study vaccines.
Among 1314 adults 50 years of age and older (Study 7) for whom the incidence of rash, urticaria, swelling, non-pitting edema, or other potential hypersensitivity reactions were actively solicited for 30 days following vaccination, a total of 2.4% of Flublok recipients and 1.6% of Comparator recipients reported such events over the 30 day follow-up period. A total of 1.9% and 0.9% of Flublok and Comparator recipients, respectively, reported these events in the 7 days following vaccination. Of these solicited events, rash was most frequently reported (Flublok 1.3%, Comparator 0.8%) over the 30 day follow-up period.
6.2 Postmarketing Experience
The following events have been spontaneously reported during post-approval use of Flublok Quadrivalent. They are described because of the temporal relationship, the biologic plausibility of a causal relationship to Flublok Quadrivalent, and their potential seriousness. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Immune system disorders: anaphylaxis, allergic reactions, and other forms of hypersensitivity (including urticaria).
7 DRUG INTERACTIONS
Data evaluating the concomitant administration of Flublok Quadrivalent with other vaccines are not available.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure
Pregnancy outcomes in women who have been exposed to Flublok Quadrivalent during pregnancy are being monitored. Sanofi Pasteur Inc. is maintaining a prospective pregnancy exposure registry to collect data on pregnancy outcomes and newborn health status following vaccination with Flublok Quadrivalent during pregnancy. Healthcare providers are encouraged to enroll women who receive Flublok Quadrivalent during pregnancy in Sanofi Pasteur Inc.'s vaccination pregnancy registry by calling 1-800-822-2463.
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively. Available data on Flublok Quadrivalent and Flublok (trivalent formulation) administered to pregnant women are insufficient to inform vaccine-associated risks in pregnant women.
There were no developmental studies of Flublok Quadrivalent formulation performed in animals. The developmental effects of Flublok (trivalent formulation) are relevant to Flublok Quadrivalent because both vaccines are manufactured using the same process and have overlapping compositions. A developmental study of Flublok (trivalent formulation) has been performed in rats administered 0.5 mL divided of Flublok (trivalent formulation) prior to mating and during gestation. This study revealed no evidence of harm to the fetus due to Flublok (trivalent formulation) [see Data].
Data
Animal
In a developmental toxicity study, female rats were administered 0.5 mL divided of Flublok (trivalent formulation) by intramuscular injection twice prior to mating (35 days and 14 days prior to mating) and on gestation Day 6. No vaccine-related fetal malformations or variations and no adverse effects on pre-weaning development were observed in the study.
8.2 Lactation
Risk Summary
It is not known whether Flublok Quadrivalent is excreted in human milk. Data are not available to assess the effects of Flublok (trivalent formulation) or Flublok Quadrivalent on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Flublok Quadrivalent and any potential adverse effects on the breastfed child from Flublok Quadrivalent or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Data from a randomized, controlled trial demonstrated that children 6 months to less than 3 years of age had diminished hemagglutinin inhibition (HI) responses to Flublok (trivalent formulation) as compared to a U.S.–licensed influenza vaccine approved for use in this population, strongly suggesting that Flublok (trivalent formulation) would not be effective in children younger than 3 years of age. Safety and effectiveness of Flublok Quadrivalent have not been established in children 3 years to less than 18 years of age.
8.5 Geriatric Use
Data from an efficacy study (Study 2), which included 1759 subjects ≥65 years and 525 subjects ≥75 years who received Flublok Quadrivalent, are insufficient to determine whether elderly subjects respond differently from younger subjects [see Clinical Trials Experience (6.1) and Clinical Studies (14)].
11 DESCRIPTION
Flublok Quadrivalent [Quadrivalent Influenza Vaccine] is a sterile, clear, colorless solution of recombinant hemagglutinin (HA) proteins from four influenza viruses for intramuscular injection. It contains purified HA proteins produced in a continuous insect cell line (expresSF+®) that is derived from Sf9 cells of the fall armyworm, Spodoptera frugiperda (which is related to moths, caterpillars and butterflies), and grown in serum-free medium composed of chemically-defined lipids, vitamins, amino acids, and mineral salts. Each of the four HAs is expressed in this cell line using a baculovirus vector (Autographa californica nuclear polyhedrosis virus), extracted from the cells with Triton X-100 and further purified by column chromatography. The purified HAs are then blended and filled into single-dose syringes.
Flublok Quadrivalent is standardized according to United States Public Health Service (USPHS) requirements. For the 2023-2024 influenza season it is formulated to contain 180 mcg HA per 0.5 mL dose, with 45 mcg HA of each of the following 4 influenza virus strains: A/West Virginia/30/2022 (A/Wisconsin/67/2022 pdm09-like virus) (H1N1), A/Darwin/6/2021 (H3N2), B/Austria/1359417/2021 and B/Phuket/3073/2013.
A single 0.5 mL dose of Flublok Quadrivalent contains sodium chloride (4.4 mg), monobasic sodium phosphate (0.2 mg), dibasic sodium phosphate (0.5 mg), and polysorbate 20 (Tween®20) (27.5 mcg). Each 0.5 mL dose of Flublok Quadrivalent may also contain residual amounts of baculovirus and Spodoptera frugiperda cell proteins (≤19 mcg), baculovirus and cellular DNA (≤10 ng), and Triton X-100 (≤100 mcg).
Flublok Quadrivalent contains no egg proteins, antibiotics, or preservatives. The single-dose, prefilled syringes contain no natural rubber latex.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Flublok Quadrivalent contains recombinant HA proteins of the four strains of influenza virus specified by health authorities for inclusion in the annual seasonal vaccine. These proteins function as antigens which induce a humoral immune response, measured by hemagglutination inhibition (HI) antibody.
Antibodies against one influenza virus type or subtype confer limited or no protection against another. Furthermore, antibodies to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent (usually annual) development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual replacement of one or more influenza virus strains in each year's influenza vaccine. Therefore, influenza vaccines are standardized to contain the hemagglutinins of influenza virus strains (i.e., typically two type A and, in quadrivalent formulations, two type B), representing the influenza viruses likely to be circulating in the U.S. in the upcoming winter.
13 NONCLINICAL TOXICOLOGY
Flublok Quadrivalent has not been evaluated for carcinogenic or mutagenic potential, or for impairment of male fertility in animals. A developmental toxicity study conducted in rats vaccinated with Flublok (trivalent formulation) revealed no evidence of impaired female fertility [see Pregnancy (8.1)].
14 CLINICAL STUDIES
14.1 Efficacy against Laboratory-Confirmed Influenza
The efficacy of Flublok (trivalent formulation) is relevant to Flublok Quadrivalent because both vaccines are manufactured using the same process and have overlapping compositions [see Description (11)].
The efficacy of Flublok (trivalent formulation) in protecting against influenza illness was evaluated in a randomized, observer-blind, placebo-controlled multicenter trial conducted in the U.S. during the 2007-2008 influenza season in adults 18-49 years of age (Study 3).
Study 3 enrolled and vaccinated 4648 healthy adults (mean age 32.5 years) randomized in a 1:1 ratio to receive a single dose of Flublok (n=2344) or saline placebo (n=2304). Among enrolled subjects, 59% were female, 67% were white, 19% African-American, 2% Asian, <1% other races, and 11% of Latino/Hispanic ethnicity. Culture-confirmed influenza was assessed by active and passive surveillance for influenza-like illness (ILI) beginning 2 weeks post-vaccination until the end of the influenza season, approximately 7 months post-vaccination. ILI was defined as having at least 2 of 3 symptoms (no specified duration) in the following categories: 1) fever ≥100ºF; 2) respiratory symptoms (cough, sore throat, or runny nose/stuffy nose); or 3) systemic symptoms (myalgias, arthralgias, headache, chills/sweats, or tiredness/malaise). For subjects with an episode of ILI, nasal and throat swab samples were collected for viral culture.
The primary efficacy endpoint of Study 3 was Centers for Disease Control-defined influenza-like illness (CDC-ILI) with a positive culture for an influenza virus strain antigenically resembling a strain represented in Flublok. CDC-ILI is defined as fever of ≥100°F oral accompanied by cough, sore throat, or both on the same day or on consecutive days. Attack rates and vaccine efficacy (VE), defined as the reduction in the influenza rate for Flublok relative to placebo, were calculated for the total vaccinated cohort (n=4648).
The pre-defined success criterion for the primary efficacy analysis was that the lower bound of the 95% confidence interval (CI) of VE should be at least 40%. Vaccine efficacy against antigenically matched culture-confirmed CDC-ILI could not be determined reliably because 96% of the influenza isolates obtained from subjects in Study 3 were not antigenically matched to the strains represented in the vaccine. An exploratory analysis of VE of Flublok against all strains, regardless of antigenic match, isolated from any subject with an ILI, not necessarily meeting CDC- ILI criteria, demonstrated an efficacy estimate of 44.8% (95% CI 24.4, 60.0). See Table 3 for a presentation of VE by case definition and antigenic similarity.
| Case definition | Flublok (trivalent) (N=2344) | Saline Placebo (N=2304) | Flublok Vaccine Efficacy†, % | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Cases, n | Rate, % | Cases, n | Rate, % | |||
|
||||||
| Positive culture with a strain represented in the vaccine | ||||||
| CDC-ILI, all matched strains‡,§ | 1 | 0.04 | 4 | 0.2 | 75.4 | (-148.0, 99.5) |
| Any ILI, all matched strains¶,# | 2 | 0.1 | 6 | 0.3 | 67.2 | (-83.2, 96.8) |
| Positive culture with any strain, regardless of match to the vaccine | ||||||
| CDC-ILI, all strains‡,Þ | 44 | 1.9 | 78 | 3.4 | 44.6 | (18.8, 62.6) |
| Sub-Type A | 26 | 1.1 | 56 | 2.4 | 54.4 | (26.1, 72.5) |
| Type B | 18 | 0.8 | 23 | 1.0 | 23.1 | (-49.0, 60.9) |
| Any ILI, all strains¶ | 64 | 2.7 | 114 | 4.9 | 44.8 | (24.4, 60.0) |
| Sub-Type A | 41 | 1.7 | 79 | 3.4 | 49.0 | (24.7, 65.9) |
| Type B | 23 | 1.0 | 36 | 1.6 | 37.2 | (-8.9, 64.5) |
Study 2 evaluated the efficacy of Flublok Quadrivalent in a randomized, observer-blind, active-controlled, multicenter trial conducted during the 2014-2015 influenza season in adults 50 years of age and older. A total of 8963 healthy, medically stable adults (mean age 62.5 years) were randomized in a 1:1 ratio to receive a single dose of Flublok Quadrivalent (n=4474) or a U.S.–licensed quadrivalent inactivated influenza vaccine (Comparator, Fluarix Quadrivalent, manufactured by Glaxo SmithKline) (n=4489). Among randomized subjects, 58% were female, 80% white, 18% black/African-American, 2% other races, and 5% of Hispanic/Latino ethnicity. A total of 5186 (60%) subjects were 50-64 years of age and 3486 (40%) were ≥65 years of age. Real-time polymerase chain reaction (rtPCR)–confirmed influenza was assessed by active and passive surveillance for influenza-like illness (ILI) beginning 2 weeks post-vaccination until the end of the influenza season, approximately 6 months post-vaccination. ILI was defined as having at least one symptom (no specified duration) in each of two categories of respiratory and systemic symptoms. Respiratory symptoms included sore throat, cough, sputum production, wheezing and difficulty breathing. Systemic symptoms included fever >99°F (>37°C) oral, chills, fatigue, headache and myalgia. For subjects with an episode of ILI, a nasopharyngeal swab sample was collected for rtPCR testing and reflex viral culture of rtPCR-positive samples.
The primary efficacy endpoint of Study 2 was rtPCR-positive, protocol-defined ILI due to any strain of influenza. Attack rates and relative vaccine efficacy (rVE), defined as 1 – (Attack rate Flublok Quadrivalent/ Attack Rate Comparator), were calculated for the total efficacy population (n=8604) for the primary efficacy endpoint and for several alternative efficacy endpoints (Table 4). Antigenic and phylogenetic evaluations of the similarity ("matching") of clinical isolates to vaccine antigens were not performed. CDC epidemiological data for the 2014-2015 influenza season indicated that Influenza A (H3N2) viruses predominated and that most influenza A/H3N2 viruses were antigenically dissimilar while A/H1N1 and B viruses were antigenically similar to vaccine antigens.
| Flublok Quadrivalent (N=4303) | Comparator (N=4301) | rVE % (95% CI) |
||||
|---|---|---|---|---|---|---|
| n | Attack Rate % (n/N) | n | Attack Rate % (n/N) | RR | ||
| Abbreviations: rtPCR=reverse transcriptase polymerase chain reaction; Comparator=U.S.–licensed quadrivalent inactivated influenza vaccine, Fluarix Quadrivalent, manufactured by GlaxoSmithKline; n=number of influenza cases; N=number of subjects in treatment group; RR=relative risk (Attack Rate Flublok/Attack Rate IIV4); rVE = ([1-RR] × 100). | ||||||
|
||||||
| All rtPCR-positive Influenza‡ | 96 | 2.2 | 138 | 3.2 | 0.70 | 30 (10, 47) |
| All rtPCR-positive Influenza A§ | 73 | 1.7 | 114 | 2.7 | 0.64 | 36 (14, 53) |
| All rtPCR-positive Influenza B§ | 23 | 0.5 | 24 | 0.6 | 0.96 | 4 (-72, 46) |
| All Culture-confirmed Protocol-defined ILI§,¶ | 58 | 1.3 | 101 | 2.3 | 0.57 | 43 (21, 59) |
14.2 Immunogenicity of Flublok Quadrivalent
Study 1 evaluated the immunogenicity of Flublok Quadrivalent as compared to a U.S.–licensed quadrivalent inactivated influenza vaccine (Comparator) (Fluarix Quadrivalent, manufactured by GlaxoSmithKline) in a randomized, observer-blind, active-controlled, multicenter trial conducted during the 2014-2015 influenza season in healthy adults 18-49 years of age. A total of 1350 subjects were enrolled, randomized 3:1, and vaccinated with Flublok Quadrivalent (998 subjects) or Comparator (332 subjects). Subjects were predominantly female (65%), white (60%), black/African American (37%), and of non-Hispanic/Latino ethnicity (84%), with a mean age of 33.5 years. Of the total vaccinated population, 1292 subjects (969 Flublok Quadrivalent and 323 IIV4 recipients, respectively) were evaluable for immune responses (Immunogenicity Population).
Post-vaccination immunogenicity was evaluated on sera obtained 28 days after administration of a single dose of study vaccine. Hemagglutination inhibition (HI) geometric mean titers (GMTs) were determined for the two vaccine groups for each vaccine antigen. Immunogenicity was compared by calculating the difference in seroconversion rates (SCR) and the ratios of GMTs of Comparator to Flublok Quadrivalent. Seroconversion was defined as either a pre-vaccination HI titer of <1:10 and a postvaccination HI titer of ≥1:40, or a pre-vaccination HI titer of ≥1:10 and a minimum 4-fold rise in postvaccination HI titer, at Day 28.
Study 1 had eight co-primary endpoints: Day 28 HI seroconversion rates and GMTs for each of the four antigens contained in the study vaccines. GMTs were compared based on the upper bound of the two-sided 95% CI of the GMT ratio of Comparator to Flublok Quadrivalent. Success in meeting this endpoint was pre-defined as an upper bound (UB) of the two-sided 95% CI of GMTComparator / GMTFlublok Quadrivalent ≤1.5. Flublok Quadrivalent met the success criterion for GMTs for three of the four antigens but not for the B/Victoria lineage antigen (Table 5).
| Antigen | Post-vaccination GMT Flublok Quadrivalent N=969 | Post-vaccination GMT Comparator N=323 | GMT Ratio Comparator/Flublok Quadrivalent [95% CI] |
|---|---|---|---|
| Abbreviations: CI, confidence interval; GMT, geometric mean titer. | |||
|
|||
| A/H1N1 | 493 | 397 | 0.81 (0.71, 0.92) |
| A/H3N2 | 748 | 377 | 0.50 (0.44, 0.57) |
| B/Yamagata | 156 | 134 | 0.86 (0.74, 0.99) |
| B/Victoria | 43 | 64 | 1.49 (1.29, 1.71) |
Success in meeting the seroconversion rate (SCR) endpoint was pre-defined as an upper bound (UB) of the two-sided 95% CI of SCR Comparator – SCR Flublok Quadrivalent ≤10%. Flublok Quadrivalent met the success criterion for SCRs for three of the four antigens but not for the B/Victoria lineage antigen (Table 6). Sub-population analyses of immunogenicity did not reveal significant differences between genders. Sub-analyses according to race and ethnicity were not informative because the sizes of the subsets were insufficient to reach meaningful conclusions. The HI response to the B/Victoria lineage antigen was low in both vaccine groups.
| Antigen | SCR (%, 95% CI) Flublok Quadrivalent N=969 | SCR (%, 95% CI) Comparator N=323 | SCR Difference (%) Comparator - Flublok Quadrivalent [95% CI] |
|---|---|---|---|
| Abbreviations: CI, confidence interval; SCR, seroconversion rate Seroconversion was defined as a pre-vaccination HI titer <1:10 and a post-vaccination HI titer ≥1:40 or a pre-vaccination HI titer ≥1:10 and a minimum four-fold rise in post-vaccination HI antibody titer. |
|||
|
|||
| A/H1N1 | 66.7 (63.6, 69.6) | 63.5 (58.0, 68.7) | -3.2 (-9.2, 2.8) |
| A/H3N2 | 72.1 (69.2, 74.9) | 57.0 (51.4, 62.4) | -15.2 (-21.3, -9.1) |
| B/Yamagata | 59.6 (56.5, 62.8) | 60.4 (54.8, 65.7) | 0.7 (-5.4, 6.9) |
| B/Victoria | 40.6 (37.4, 43.7) | 58.2 (52.6, 63.6) | 17.6 (11.4, 23.9) |
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Inform the vaccine recipient of the potential benefits and risks of vaccination with Flublok Quadrivalent.
Inform the vaccine recipient that:
- Flublok Quadrivalent contains non-infectious proteins that cannot cause influenza.
- Flublok Quadrivalent stimulates the immune system to produce antibodies that help protect against the influenza viruses carrying the proteins contained in the vaccine, but does not prevent other respiratory infections.
Instruct the vaccine recipient to report any adverse events to their healthcare provider and/or to the Vaccine Adverse Event Reporting System (VAERS).
Provide the vaccine recipient with the Vaccine Information Statements which are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to vaccination. These materials are available free of charge at the Centers for Disease Control (CDC) website (www.cdc.gov/vaccines).
Encourage women who receive Flublok or Flublok Quadrivalent while pregnant to notify Sanofi Pasteur Inc. by calling 1-800-822-2463.
Instruct the vaccine recipient that annual vaccination to prevent influenza is recommended.
Manufactured by Protein Sciences Corporation (Meriden, CT).
U.S. license No. 1795.
Distributed by Sanofi Pasteur Inc.
Flublok is a registered trademark of Protein Sciences Corporation.
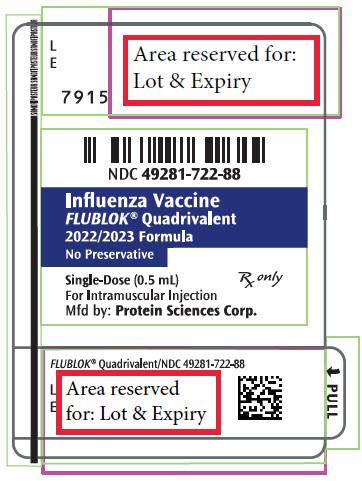
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label - 7915
NDC 49281-722-88
Influenza Vaccine
FLUBLOK® Quadrivalent
2022/2023 Formula
No Preservative
Single-Dose (0.5 mL)
Rx only
For Intramuscular Injection
Mfd by: Protein Sciences Corp.
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Carton - 7916
NDC 49281-722-10
Influenza Vaccine
FLUBLOK® Quadrivalent
2022 / 2023 Formula
10 Single-Dose
Prefilled Syringes
0.5 mL each
Rx only
For 18 Years of Age and Older
For Intramuscular Administration Only
SANOFI PASTEUR

PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label - CP-1931
NDC 49281-722-88
Influenza Vaccine
FLUBLOK® Quadrivalent
2022/2023 Formula
No Preservative
Single-Dose (0.5 mL)
Rx only
For Intramuscular Injection
Mfd by: Protein Sciences Corp.
CP-1931

PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Carton - CP-1930
NDC 49281-722-10
Influenza Vaccine
FLUBLOK® Quadrivalent
2022 / 2023 Formula
10 Single-Dose
Prefilled Syringes
0.5 mL each
Rx only
For 18 Years of Age and Older
For Intramuscular Administration Only
SANOFI PASTEUR

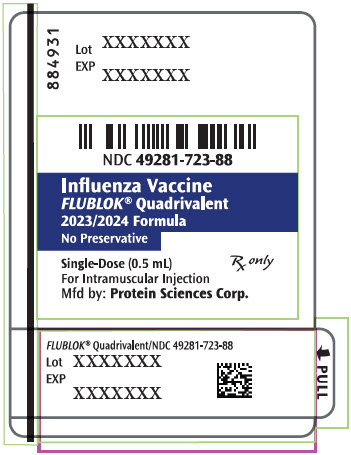
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label - 884931
NDC 49281-723-88
Influenza Vaccine
FLUBLOK® Quadrivalent
2023/2024 Formula
No Preservative
Single-Dose (0.5 mL)
Rx only
For Intramuscular Injection
Mfd by: Protein Sciences Corp.
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Carton - 884932
NDC 49281-723-10
Influenza Vaccine
FLUBLOK® Quadrivalent
2023 / 2024 Formula
10 Single-Dose
Prefilled Syringes
0.5 mL each
Rx only
For 18 Years of Age and Older
For Intramuscular Administration Only
SANOFI PASTEUR


