DOSAGE & ADMINISTRATION:
Prior to use, the applicator stick may be bent or shaped to allow easier access to the target area. The
medicated end of the applicator should be moistened in clean water and applied to the affected tissue.
One applicator is normally sufficient for each application. The strength of the action is controlled by
the amount of water used to moisten the tip. The applicator action can be stopped by washing the area
with a saline solution (0.9% sodium chloride).
USES:
Caustic applicators are useful for cauterization of skin or mucous membrane and for the removal of
granulation tissue, warts and verrucae.
CONTRAINDICATIONS & ANTIDOTES:
Caustic applicators must not be used for genital warts. Continued application to mucous membranes and
open wounds leads to argyria, a bluish-black discoloration of the skin due to depositions of granules of
silver compounds in the connective tissues.
This condition persists indefinitely or disappears very slowly. Poisoning by oral ingestion is unlikely
with the quantities involved, but treatment for poisoning is the immediate ingestion of large amounts of
salt water followed by an emetic. Then administer a dose of Epsom Salts followed with milk.
Immediately call a physician.
CAUTION:
Federal (USA) law prohibits dispensing without prescription.
Keep out of reach of children.
Do not use on or near eyes. Do not use on genital warts.
Avoid storage in direct sunlight/fluorescent lighting and store in a cool, dry, and well-ventilated area.
Store at room temperature in the closed package, in a dry place protected from light. Exposure to light
will cause the silver nitrate tip to turn black, but will not affect the product's potency.
Not made with natural rubber latex.
Questions? Call 1-800-777-4908
Distributed by McKesson Medical-Surgical Inc.
Richmond, VA 23233
PVN A0417
Made in England
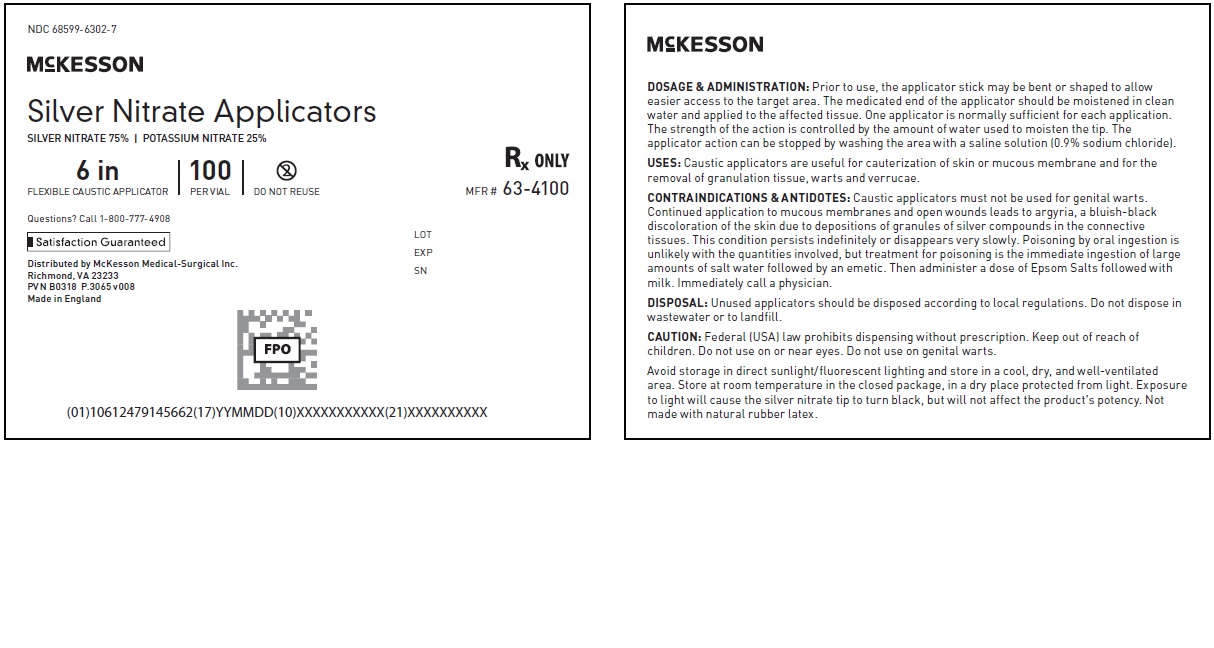
NDC 68599-6302-7
McKesson
Silver Nitrate Applicators MFR # 63-4100
SILVER NITRATE 75% | POTASSIUM NITRATE 25%
6 in
FLEXIBLE CAUSTIC APPLICATOR
100
PER VIAL
DO NOT REUSE
Questions? Call 1-800-777-4908
Distributed by McKesson Medical-Surgical Inc.
Richmond, VA 23233
PVN B0118 P.3065 v008
Made in England