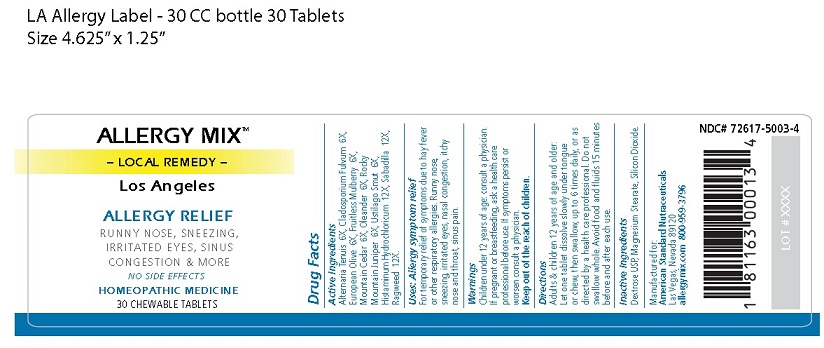
Active Ingredients
Alternaria Tenuis 6X, Cladosporium Fulvum 6X,
European Olive 6X, Fruitless Mulberry 6X,
Mountain Cedar 6X, Oleander 6X, Rocky
Mountain Juniper 6X, Ustilago Smut 6X,
Histaminum Hydrochloricum 12X, Sabadilla 12X,
Ragweed 12X.
Uses: Allergy symptom relief
For temporary relief of symptoms due to hay fever
or other respiratory allergies. Runny nose,
sneezing, irritated eyes, nasal congestion, itchy
nose and throat, sinus pain.
Warnings
Children under 12 years of age: consult a physician.
If pregnant or breastfeeding, ask a health care
professional before use. If symptoms persist or
worsen consult a physician.
Directions
Adults & children 12 years of age and older:
Let one tablet dissolve slowly under tongue or
chew, then swallow, up to 6 times daily, or as
directed by a health care professional. Do not
swallow whole. Avoid food and fluids 15 minutes
before and after each use.
Principal Display Package
ALLERGY MIX™
– LOCAL REMEDY –
Los Angeles
ALLERGY RELIEF
RUNNY NOSE, SNEEZING,
IRRITATED EYES, SINUS
CONGESTION & MORE
NO SIDE EFFECTS
HOMEOPATHIC MEDICINE
30 CHEWABLE TABLETS
NDC# 72617-5003-4
Manufactured for:
American Standard Nutraceuticals
Las Vegas, Nevada 89120
allergymix.com 800-959-3796
1 81163 00013 4
LOT # XXXX
res